S8139
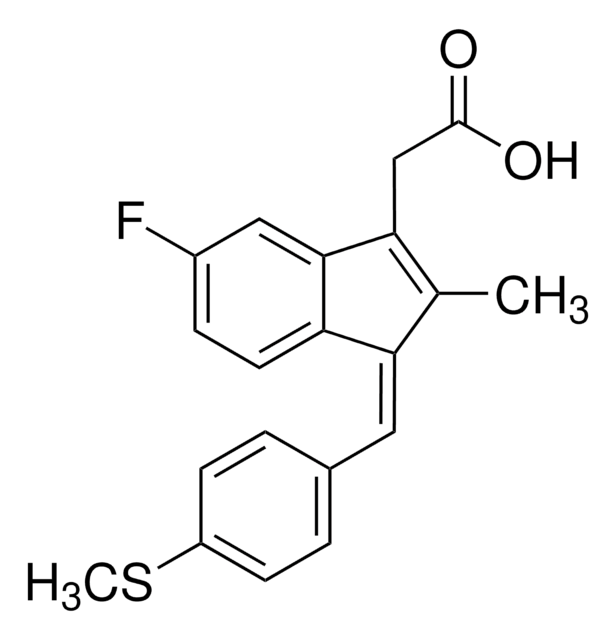
Sulindac
≥98.0%
Synonyme(s) :
(Z)-5-Fluoro-2-methyl-1-[p-(methylsulfinyl)benzylidene]indene-3-acetic acid
About This Item
Produits recommandés
Source biologique
synthetic (organic)
Pureté
≥98.0%
Forme
powder
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Solubilité
methanol: 50 mg/mL
Application(s)
forensics and toxicology
veterinary
Auteur
Merck & Co., Inc., Kenilworth, NJ, U.S.
Chaîne SMILES
CC1=C(CC(O)=O)c2cc(F)ccc2\C1=C/c3ccc(cc3)S(C)=O
InChI
1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9-
Clé InChI
MLKXDPUZXIRXEP-MFOYZWKCSA-N
Informations sur le gène
human ... ALB(213) , PTGS1(5742) , PTGS2(5743)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral - Repr. 2 - Resp. Sens. 1 - Skin Sens. 1
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Protein-based drug transporters are expressed in Sf9 cells. Understanding the specific mechanisms of tumor cell transporters is an essential aspect of chemotherapeutic drug design.
Discover Bioactive Small Molecules for ADME/Tox
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique