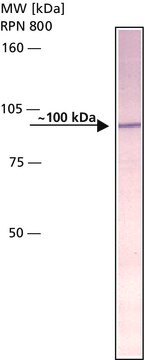
S4316
Amyloid Precursor Protein β, Secreted human
recombinant, expressed in E. coli (N-terminal histidine tagged), solution
Synonyme(s) :
Amyloid Precursor Protein β, Secreted, sAPPβ
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Description générale
Amyloid-β (Aβ) peptides are a major component of the senile plaques characteristic of the Alzheimer brain. It is a type- I transmembrane protein. The gene encoding amyloid-β is localized on human chromosome 21.
Application
Human amyloid precursor protein β has been used as peptide standard.
Actions biochimiques/physiologiques
The secreted amyloid precursor β (sAPPβ) is a proteolytic cleavage product of β amyloid precursor protein (APP). APP is cleaved by β-secretase into two fragments, sAPPβ and β-amyloid peptide (Aβ). The Aβ is a component of amyloid plaques, one of the major hallmarks of Alzheimer`s disease (AD), while the sAPPβ is thought to modulate neuronal function and cell survival. Mutation in APP found in Swedish familial AD pedigree increases the susceptibility of APP to β-secretase cleavage and the formation of Aβ and sAPPβ. In contrast, activation of protein kinase C (PKC) decreases the levels of sAPPβ. The trans-Golgi network (TGN) is the major site for β-secretase activity.
Forme physique
0.2 μm filtered solution in 20 mM Tris (pH 7.4), 0.5 M NaCl, 1 mM EDTA, 5 mM βME, 2 μg/ml aprotinin
Notes préparatoires
sAPPβ was expressed in E. coli as a soluble protein and purified under non-denaturing conditions
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
D J Selkoe
Nature, 399(6738 Suppl), A23-A31 (1999-07-07)
Studies of the molecular basis of Alzheimer's disease exemplify the increasingly blurred distinction between basic and applied biomedical research. The four genes so far implicated in familial Alzheimer's disease have each been shown to elevate brain levels of the self-aggregating
Regulation of global gene expression and cell proliferation by APP.
Wu Y
Scientific Reports (2016)
Conformational Dynamics of Specific A? Oligomers Govern Their Ability To Replicate and Induce Neuronal Apoptosis.
Dean DN
Biochemistry (2016)
In vivo BACE1 inhibition leads to brain A? lowering and increased a-secretase processing of APP without effect on Neuregulin-1
Sethu Sankaranarayanan
Journal of Pharmacology and Experimental Therapeutics (2018)
D M Skovronsky et al.
The Journal of biological chemistry, 275(4), 2568-2575 (2000-01-25)
The release of amyloidogenic amyloid-beta peptide (Abeta) from amyloid-beta precursor protein (APP) requires cleavage by beta- and gamma-secretases. In contrast, alpha-secretase cleaves APP within the Abeta sequence and precludes amyloidogenesis. Regulated and unregulated alpha-secretase activities have been reported, and the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique