CC123
Caspase 8, recombinant human active
Synonyme(s) :
FLICE, MASH, Mch5
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352200
eCl@ss :
32160405
Nomenclature NACRES :
NA.41
Produits recommandés
Source biologique
human
Produit recombinant
expressed in E. coli
Forme
solid
Activité spécifique
25 units
Fabricant/nom de marque
Chemicon®
Technique(s)
activity assay: suitable
Numéro d'accès NCBI
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Description générale
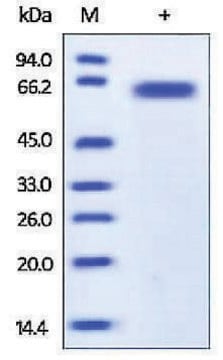
Caspase-8 is a member of the interleukin-1β converting enzyme (ICE) family of cysteine proteases. As with other caspases, caspase-8 also exists in cells as an inactive proenzyme. During apoptosis procaspase-8 is processed at aspartate residues by self-proteolysis and/or cleavage by another caspase. The processed form of caspase-8 consists of large and small subunits which associate to form the active enzyme. The active caspase-8 has been shown to be involved in the proteolysis of poly (ADP-ribose) polymerase (PARP), an enzyme that is involved in DNA repair and genomic maintenance.
The active recombinant human caspase-8 was expressed in E. coli. The expressed caspase-8 spontaneously undergoes autoprocessing to yield the subunits characteristic of the active enzyme. The recombinant caspase-8 preferentially cleaves the substrates consisting of consensus sequence IETD (e.g., IETD-AFC and IETD-pNA). The recombinant enzyme can also be used as a positive control in caspase assays or in determining the specificty of caspase substrates.Recommended usage: 0.5-1 unit for fluorometric caspase assays and 2-4 units for colorimetric caspase assays. Optimal working dilutions must be determined by end user.
Application
Recommended usage: 0.5-1 unit for fluorometric caspase assays and 2-4 units for colorimetric caspase assays.
Optimal working dilutions must be determined by end user.
Optimal working dilutions must be determined by end user.
Définition de l'unité
Specific Activity: Where one unit of the recombinant caspase-8 is the enzyme activity that cleaves 1 nmol of the caspase substrate IETD-pNA (pNA: pnitroanaline) per hour at 37°C in a reaction solution containing 50 mM HEPES, 50 mM NaCl, 0.1% CHAPS, 10 mM EDTA, 5% Glycerol, and 10 mM DTT.
Forme physique
Lyophilized. Reconstitute with 25 μl PBS containing 15% glycerol.
Stockage et stabilité
Maintain at -70°C for 3-6 months. Avoid repeated freeze/thaw cycles.
Informations légales
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Clause de non-responsabilité
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Specific caspase pathways are activated in the two stages of cerebral infarction.
A Benchoua,C Guegan,C Couriaud,H Hosseini,N Sampaio,D Morin,B Onteniente
The Journal of Neuroscience null
Jiro Fujita et al.
Microbiology and immunology, 47(6), 447-451 (2003-08-09)
In a previous study, we demonstrated anti-vimentin antibodies in sera of patients with interstitial pneumonia. We hypothesized that antibodies in sera might detect vimentin fragments formed during the process of apoptosis. To prove this, recombinant human vimentin was digested by
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique