850398C
Avanti
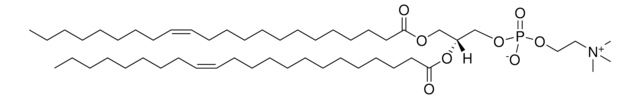
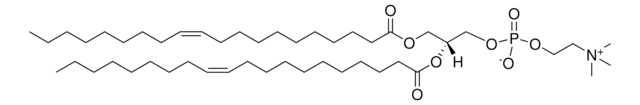
22:1 (Cis) PC
1,2-dierucoyl-sn-glycero-3-phosphocholine, chloroform
Synonyme(s) :
1,2-di-(13Z-docosenoyl)-sn-glycero-3-phosphocholine; PC(22:1(13Z)/22:1(13Z)); DEPC
About This Item
Produits recommandés
Essai
>99% (TLC)
Forme
liquid
Conditionnement
pkg of 1 × 2.5 mL (850398C-25mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 850398C
Concentration
10 mg/mL (850398C-25mg)
Type de lipide
phospholipids
cardiolipins
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
[P](=O)([O-])(OC[C@H](OC(=O)CCCCCCCCCCC\C=C\CCCCCCCC)COC(=O)CCCCCCCCCCC\C=C\CCCCCCCC)OCC[N+](C)(C)C
InChI
1S/C52H100NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-38-40-42-44-51(54)58-48-50(49-60-62(56,57)59-47-46-53(3,4)5)61-52(55)45-43-41-39-37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h20-23,50H,6-19,24-49H2,1-5H3/b22-20+,23-21+/t50-/m1/s1
Clé InChI
SDEURMLKLAEUAY-QVOKQPLLSA-N
Description générale
Application
Actions biochimiques/physiologiques
Conditionnement
Informations légales
Souvent commandé avec ce produit
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organes cibles
Central nervous system, Liver,Kidney
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique