PZ0241
PF-6422899
≥98% (HPLC)
Sinônimo(s):
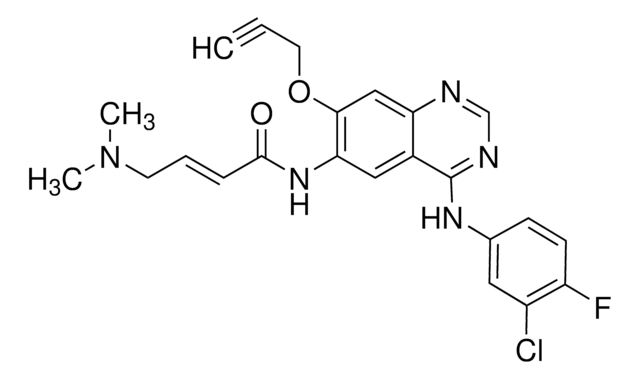
N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-(2-propyn-1-yloxy)-6-quinazolinyl]-2-propenamide
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98% (HPLC)
forma
powder
cor
white to beige
solubilidade
DMSO: 5 mg/mL, clear (warmed)
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
FC(C=C1)=C(Cl)C=C1NC2=NC=NC3=CC(OCC#C)=C(NC(C=C)=O)C=C32
InChI
1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25)
chave InChI
AZLXGJFHSLQCPV-UHFFFAOYSA-N
Ações bioquímicas/fisiológicas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Conteúdo relacionado
The aim of the Cravatt research group is to understand the roles that mammalian enzymes play in physiological and pathological processes and to use this knowledge to identify novel therapeutic targets for the treatment of human disease. To achieve these goals, they develop and apply new technologies that bridge the fields of chemistry and biology, ascribing to the philosophy that the most significant biomedical problems require creative multidisciplinary approaches for their solution. The group's technological innovations address fundamental challenges in systems biology that are beyond the scope of contemporary methods. For instance, enzymes are tightly regulated by post-translational events in vivo, meaning that their activity may not correlate with expression as measured by standard genomic and proteomic approaches. Considering that it is an enzyme's activity, rather than abundance that ultimately dictates its role in cell physiology and pathology, the Cravatt group has introduced a set of proteomic technologies that directly measures this parameter. These activity-based protein profiling (ABPP) methods exploit the power of chemistry to engender new tools and assays for the global analysis of enzyme activities. The enzyme activity profiles generated by ABPP constitute unique molecular portraits of cells and tissues that illuminate how metabolic and signaling networks are regulated in vivo. Additionally, by evaluating enzymes based on functional properties rather than mere abundance, ABPP acquires high-content proteomic information that is enriched in novel markers and targets for the diagnosis and treatment of human disease.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica