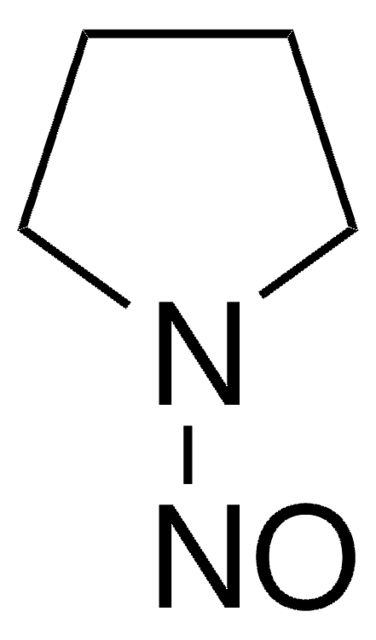
158240
1-Nitrosopyrrolidine
99%
Sinônimo(s):
N-Nitrosopyrrolidine, NPYR
About This Item
Produtos recomendados
Ensaio
99%
índice de refração
n20/D 1.489 (lit.)
pb
214 °C (lit.)
densidade
1.085 g/mL at 25 °C (lit.)
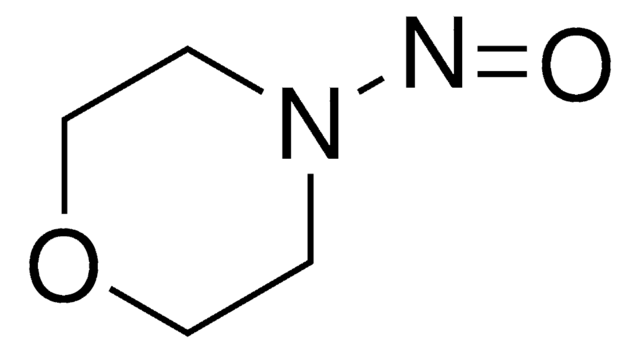
cadeia de caracteres SMILES
O=NN1CCCC1
InChI
1S/C4H8N2O/c7-5-6-3-1-2-4-6/h1-4H2
chave InChI
WNYADZVDBIBLJJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Ações bioquímicas/fisiológicas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Carc. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
208.4 °F - closed cup
Ponto de fulgor (°C)
98 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica