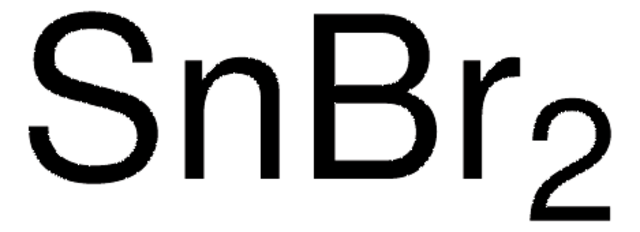204722
Tin(II) chloride
≥99.99% trace metals basis
Sinônimo(s):
Stannous chloride
About This Item
Produtos recomendados
pressão de vapor
33 hPa (~429 °C)
Nível de qualidade
Ensaio
≥99.99% trace metals basis
forma
crystalline powder
flakes
adequação da reação
reagent type: catalyst
core: tin
pH
2.18 (20 °C)
pb
652 °C (lit.)
pf
246 °C (lit.)
cadeia de caracteres SMILES
Cl[SnH2]Cl
InChI
1S/2ClH.Sn/h2*1H;/q;;+2/p-2
chave InChI
AXZWODMDQAVCJE-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
- aromatic nitro compounds
- nitriles
- cyanosilyl ethers
- organic azides
Aplicação
- To catalyze the addition of diazo sulfones, diazo phosphine oxides and diazo phosphonates to aldehydes to form β-keto sulfones, β-keto phosphine oxides and β-keto phosphonates, respectively.
- Along with trityl chloride, to catalyze the aldol reaction of silyl enol ethers with acetals or aldehydes and the Michael reaction of silyl enol ethers with α,β-unsaturated ketones.
- As a promoter in the allylic amination of allylic alcohols with amines in the presence of palladium catalyst.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 Oral - STOT SE 3
Órgãos-alvo
Cardio-vascular system, Respiratory system
Código de classe de armazenamento
8B - Non-combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificados de análise (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica