19667
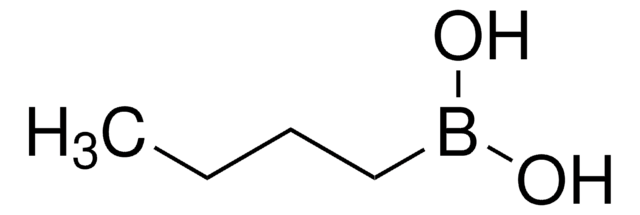
Butylboronic acid
for GC derivatization, LiChropur™, ≥96.0% (T)
Sinônimo(s):
1-Butaneboronic acid
About This Item
Produtos recomendados
grau
for GC derivatization
Nível de qualidade
Ensaio
≥96.0% (T)
qualidade
LiChropur™
adequação da reação
reagent type: derivatization reagent
reaction type: Alkylations
técnica(s)
gas chromatography (GC): suitable
pf
90-92 °C (lit.)
90-92 °C
cadeia de caracteres SMILES
CCCCB(O)O
InChI
1S/C4H11BO2/c1-2-3-4-5(6)7/h6-7H,2-4H2,1H3
chave InChI
QPKFVRWIISEVCW-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Developing a reference measurement procedure for free glycerol in human serum by two-step gas chromatography-isotope dilution mass spectrometry.: This research employs butylboronic acid in a reference measurement procedure to quantify free glycerol in human serum. The method is significant for clinical diagnostics, providing accurate and reliable measurements for metabolic studies (Chen et al., 2015).
Outras notas
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificados de análise (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica