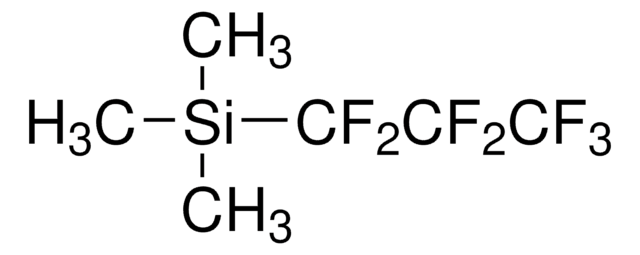900015
Trimethylpentafluoroethylsilane
97%
Sinônimo(s):
(Pentafluoroethyl)trimethylsilane
About This Item
Produtos recomendados
Ensaio
97%
forma
liquid
índice de refração
n/D 1.325
densidade
1.095 g/mL
cadeia de caracteres SMILES
C[Si](C)(C)C(F)(F)C(F)(F)F
InChI
1S/C5H9F5Si/c1-11(2,3)5(9,10)4(6,7)8/h1-3H3
chave InChI
MTPVUVINMAGMJL-UHFFFAOYSA-N
Descrição geral
Aplicação
- 5-bromo-2-(perfluoroethyl)quinoline
- 8-methoxy-2-(perfluoroethyl)quinoline
- 1-(perfluoroethyl)isoquinoline
- 8-(tert-butoxy)-5,7-dichloro-2-(perfluoroethyl)quinolone
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
1.4 °F - closed cup - (calculated)
Ponto de fulgor (°C)
-17 °C - closed cup - (calculated)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Conteúdo relacionado
The major research interests of Prof. Jinbo Hu's lab include the development of new fluorination reagents and reactions, especially the difluoromethylation, difluoromethylenation, and monofluoromethylation methods.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica