About This Item
Fórmula empírica (Notação de Hill):
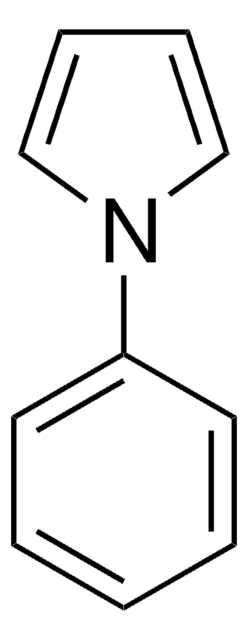
C11H11N
Número CAS:
Peso molecular:
157.21
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
índice de refração
n20/D 1.5685 (lit.)
p.e.
90 °C/0.9 mmHg (lit.)
densidade volumétrica
1.020 g/mL
cadeia de caracteres SMILES
C(c1ccccc1)n2cccc2
InChI
1S/C11H11N/c1-2-6-11(7-3-1)10-12-8-4-5-9-12/h1-9H,10H2
chave InChI
FNEQHKCQXDKYEO-UHFFFAOYSA-N
Descrição geral
N-benzylpyrrole can be synthesized from N-benzylpyrrolidine via oxidation with 2-iodoxybenzoic acid(IBX) in presence of β-cyclodextrin in aqueous medium.
Aplicação
N-benzylpyrrole may be used in the synthesis of the following:
- (E,Z)-3-(7,8-dimethoxy-5H-pyrrolo[2,1-a]isoindol-3-yl)-N,N-diethylacrylamide
- (E,Z)-7,8-dimethoxy-3-styryl-5H-pyrrolo[2,1-a]isoindole
- pyrroloisoquinolines
- (Z)-2-(7,8-dimethoxypyrrolo[1,2-b]isoquinolin-10-ylidene)-N,N-diethylacetamide
- (E,Z)-10-benzylidene-7,8-dimethoxy-5,10-dihydropyrrolo- [1,2-b]isoquinoline
- (E,Z)-7,8-dimethoxy-10-(2-methoxyvinyl)-5,10-dihydropyrrolo[1,2-b]isoquinoline
- (Z)-7,8-dimethoxy-10-(methoxymethylene)-5,10-dihydropyrrolo[1,2-b]isoquinoline
- 2-cyano-N-benzylpyrroles
- 3-cyano-N-benzylpyrroles
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
"Metal-Free One-Pot Conversion of Electron-Rich Aromatics into Aromatic Nitriles"
Ushijima S and Togo H
Synlett, 2010(07), 1067-1070 (2010)
"o-Iodoxybenzoic acid (IBX): a versatile reagent for the synthesis of N-substituted pyrroles mediated by ?-cyclodextrin in water"
Murthy N.S and Nageswar DVY
Tetrahedron Letters, 52(34), 4481-4484 (2001)
"Intramolecular Palladium-Catalyzed Direct Arylation vs. Heck Reactions: Synthesis of Pyrroloisoquinolines and Isoindoles"
Lage S, et al.
Advanced Synthesis & Catalysis, 351(14 - 15), 2460-2468 (2009)
Hannah F Dugdale et al.
Molecular and cellular biochemistry, 444(1-2), 109-123 (2017-12-01)
Glucose restriction (GR) impairs muscle cell differentiation and evokes myotube atrophy. Resveratrol treatment in skeletal muscle cells improves inflammatory-induced reductions in skeletal muscle cell differentiation. We therefore hypothesised that resveratrol treatment would improve muscle cell differentiation and myotube hypertrophy in
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica