513830
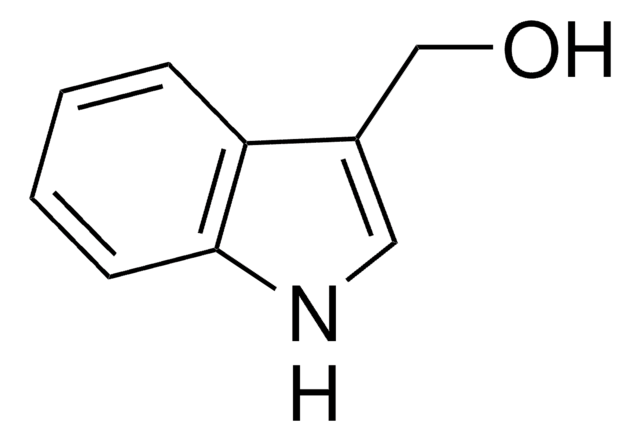
Indole-5-carboxaldehyde
98%
Sinônimo(s):
5-Formylindole
About This Item
Produtos recomendados
Ensaio
98%
pf
100-103 °C (lit.)
cadeia de caracteres SMILES
O=Cc1ccc2[nH]ccc2c1
InChI
1S/C9H7NO/c11-6-7-1-2-9-8(5-7)3-4-10-9/h1-6,10H
chave InChI
ADZUEEUKBYCSEY-UHFFFAOYSA-N
Aplicação
- Preparation of curcumin derivatives as anti-proliferative & anti-inflammatory agents
- Preparation of analogs of botulinum neurotoxin serotype A protease inhibitors
- Stereoselective synthesis of dibenzylideneacetone derivatives as β-amyloid imaging probes
- Synthesis of para-para stilbenophanes by McMurry coupling
- Stereoselective synthesis of heteroaromatic (E)-α,β-unsaturated ketones from aldehydes
- Structure-based drug design of aurora kinase A inhibitors
- Preparation of 5-indolyl linked 15- and 18-membered azacrown ethers to study their cation-π interactions.
- Preparation of bibenzimidazole derivatives substituted 5-indolyl moiety in the study of inhibition of topoisomerase I activity.
- Synthesis of (5-(4-(3,4,5-trimethoxybenzoyl)-1H-imidazol-2-yl)-1H-indol-2-yl)(3,4,5-trimethoxyphenyl)methanone and radioiodinated indolochalcone.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica