About This Item
Fórmula linear:
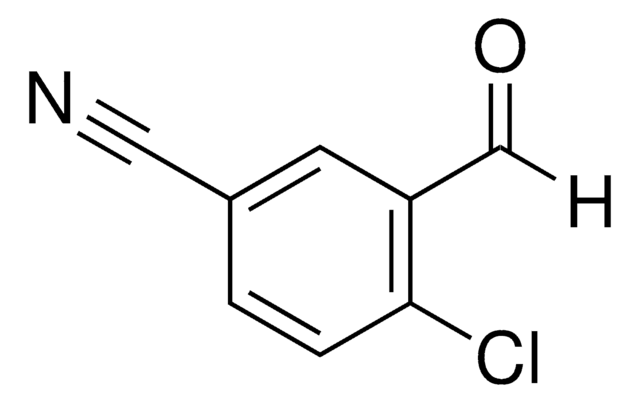
ClC6H3(F)CN
Número CAS:
Peso molecular:
155.56
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
pf
69-71 °C (lit.)
grupo funcional
chloro
fluoro
nitrile
cadeia de caracteres SMILES
Fc1ccc(cc1Cl)C#N
InChI
1S/C7H3ClFN/c8-6-3-5(4-10)1-2-7(6)9/h1-3H
chave InChI
VAHXXQJJZKBZDX-UHFFFAOYSA-N
Descrição geral
The FTIR and Raman spectra of 3-chloro-4-fluorobenzonitrile by ab initio HF and density functional method has been reported. Reaction of 3-chloro-4-fluorobenzonitrile with potassium fluoride in 1,3-dimethylimidazolidine-2-one (DMI) has been reported.
Aplicação
3-Chloro-4-fluorobenzonitrile may be used in the preparation of ethyl 5-(2-chloro-4-cyanophenoxy)indoline-1-carboxylate and 3-chloro-4-(indolin-5-yloxy)benzonitrile.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 1134-1139 (2008-05-02)
In this work, the experimental and theoretical spectra of 3-chloro-4-fluoro benzonitrile (3C4FBN) were studied. The Fourier transform infrared and Fourier transform Raman spectra of 3C4FBN were recorded in the solid phase. The optimized geometry was calculated by HF and B3LYP
S Igarashi et al.
Chemical & pharmaceutical bulletin, 48(11), 1689-1697 (2000-11-22)
While searching for novel nonsteroidal inhibitors of human and rat prostatic 5alpha-reductases, we found a new series of indoline and aniline derivatives that showed potent inhibitory activities for both enzymes. Among them, 3-chloro-4-¿[1-(4-phenoxybenzyl)indolin-5-yl]oxylbenzoic acid (2e, YM-36117) showed a more potent
Synthesis of 3, 4-difluorobenzonitrile and monofluorobenzonitriles by means of halogen-exchange fluorination.
Suzuki H and Kimura Y.
Journal of Fluorine Chemistry, 52(3), 341-351 (1991)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica