If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
374733
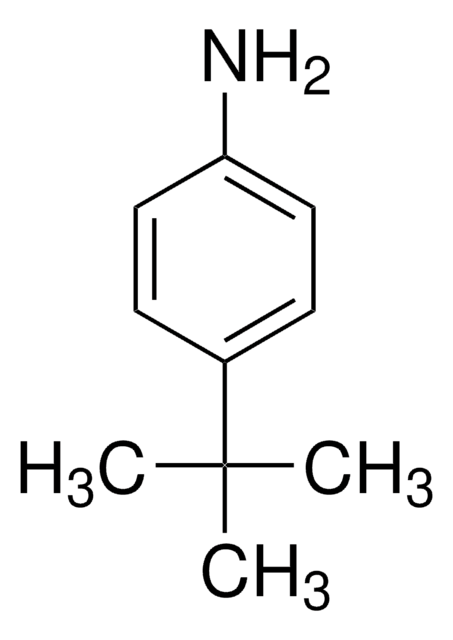
2,6-Diisopropylaniline
97%
Sinônimo(s):
2,6-Bis(1-methylethyl)benzenamine, 2,6-Bis(propan-2-yl)aniline, 2,6-Diisopropylphenylamine
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
pressão de vapor
<0.01 mmHg ( 20 °C)
Nível de qualidade
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.532 (lit.)
p.e.
257 °C (lit.)
pf
−45 °C (lit.)
densidade
0.94 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
CC(C)c1cccc(C(C)C)c1N
InChI
1S/C12H19N/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9H,13H2,1-4H3
chave InChI
WKBALTUBRZPIPZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.[2]
[3]2,6-Diisopropylaniline is an important organic intermediate widely used to synthesize plastics and dyes.[2]
Aplicação
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 3 - Eye Irrit. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica