About This Item
Fórmula empírica (Notação de Hill):
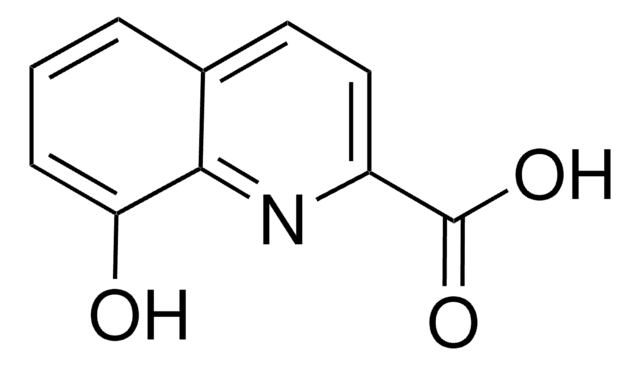
C10H7NO2
Número CAS:
Peso molecular:
173.17
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
pf
183-185 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
OC(=O)c1cccc2cccnc12
InChI
1S/C10H7NO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6H,(H,12,13)
chave InChI
QRDZFPUVLYEQTA-UHFFFAOYSA-N
Descrição geral
Herbicide 8-quinolinecarboxylic acid and its removal from aqueous solution using sodium montmorillonite, acidic montmorillonite and organo-acidic montmorillonite has been reported.
Aplicação
8-Quinolinecarboxylic acid may be used in the synthesis of:
- novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives
- chiral 1,2,3,4-tetrahydroquinolinyl-oxazoline compounds, used as ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones
- chiral quinolinyl-oxazoline compounds, used as ligands for Cu(II) catalyzed asymmetric cyclopropanation
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
S López-Bernabeu et al.
Chemosphere, 144, 982-988 (2015-10-05)
The effect of the electrochemical treatment (potentiostatic treatment in a filter-press electrochemical cell) on the adsorption capacity of an activated carbon cloth (ACC) was analyzed in relation with the removal of 8-quinolinecarboxylic acid pollutant from water. The adsorption capacity of
Chiral quinolinyl-oxazolines as ligands for copper (I)-catalyzed asymmetric cyclopropanation.
Wu X-Y, et al.
Tetrahedron Asymmetry, 9(23), 4143-4150 (1998)
Chiral 1, 2, 3, 4-tetrahydroquinolinyl-oxazoline ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones.
Zhou Y-B, et al.
Tetrahedron Asymmetry, 13(5), 469-473 (2002)
Barbara Machura et al.
Dalton transactions (Cambridge, England : 2003), 42(24), 8827-8837 (2013-05-04)
Six novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives were prepared in good yields. Relying on the experimental conditions, compounds with two chelate ligands [ReOCl(iqc)2]·MeOH (1), [ReO(OMe)(iqc)2] (2), [ReO(OMe)(mqc)2] (3) and [ReO(OMe)(8-qc)2] (4) and compounds incorporating one bidentate
M Mekhloufi et al.
Environmental monitoring and assessment, 185(12), 10365-10375 (2013-08-09)
Sodium montmorillonite (Na-M), acidic montmorillonite (H-M), and organo-acidic montmorillonite (Org-H-M) were applied to remove the herbicide 8-quinolinecarboxylic acid (8-QCA). The montmorillonites containing adsorbed 8-QCA were investigated by transmission electron microscopy, FT-IR spectroscopy, X-ray diffraction analysis, X-ray fluorescence thermogravimetric analysis, and
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica