161195
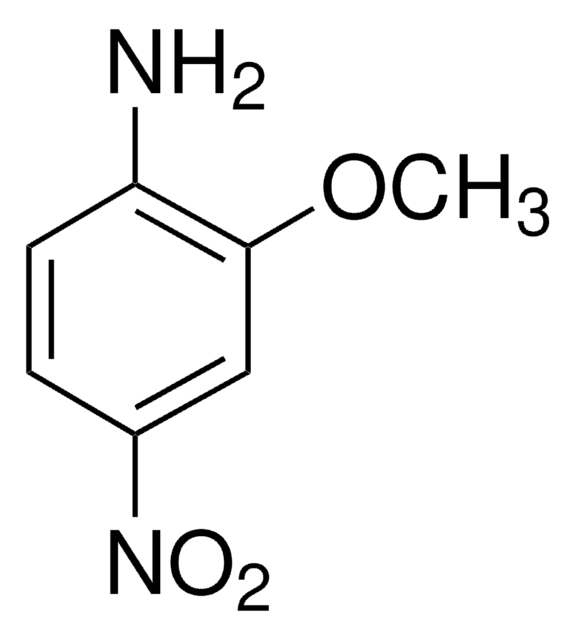
2-Methoxy-5-nitroaniline
98%
Sinônimo(s):
5-Nitro-o-anisidine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3OC6H3(NO2)NH2
Número CAS:
Peso molecular:
168.15
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
pf
117-119 °C (lit.)
cadeia de caracteres SMILES
COc1ccc(cc1N)[N+]([O-])=O
InChI
1S/C7H8N2O3/c1-12-7-3-2-5(9(10)11)4-6(7)8/h2-4H,8H2,1H3
chave InChI
NIPDVSLAMPAWTP-UHFFFAOYSA-N
Descrição geral
2-Methoxy-5-nitroaniline is an aromatic metabolite of 2,4-dinitroanisole.
Aplicação
2-Methoxy-5-nitroaniline was used in the synthesis of 5-(9-acridinylamino)-p-anisidines via reaction with 9-anilinoacridines. It was also used in the synthesis of disazo disperse dyes containing nitro and methoxy groups, used for the dyeing of polyester fibre.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
246.2 °F - closed cup
Ponto de fulgor (°C)
119 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Jidong Liang et al.
Journal of hazardous materials, 262, 281-287 (2013-09-18)
2,4-Dinitroanisole (DNAN) is an insensitive munitions compound considered to replace conventional explosives such as 2,4,6-trinitrotoluene (TNT). DNAN undergoes facile microbial reduction to 2-methoxy-5-nitroaniline (MENA) and 2,4-diaminoanisole (DAAN). This study investigated the inhibitory effect of DNAN, MENA, and DAAN toward various
Disperse dyes derived from 2-methoxy-5-nitroaniline.
Otutu JO and Osabohien E.
Orient. J. Chem., 25(4), 863-863 (2009)
A Dewanji et al.
Biometrics, 49(2), 367-377 (1993-06-01)
In this paper, a new method of estimating tumorigenic potency is proposed that takes into account information on survival and, when available, the underlying cause of death. Specifically, Weibull distributions are used to describe the time to tumor occurrence (X)
Valeriy A Bacherikov et al.
Bioorganic & medicinal chemistry, 13(23), 6513-6520 (2005-09-06)
A series of 5-(9-acridinylamino)anisidines were synthesized by condensing methoxy-substituted 1,3-phenylenediamines (10 and 11) with 9-chloroacridine derivatives to form 5-(9-acridinylamino)-m-anisidines (AMAs, 14a-e) and 5-(9-acridinylamino)-o-anisidines (AOAs, 15a-e). 5-(9-Acridinylamino)-p-anisidines (APAs, 17a-e) were synthesized by reacting 2-methoxy-5-nitroaniline (12) with 9-anilinoacridines, followed by reduction. The
5-Nitro-ortho-anisidine.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 27, 133-139 (1982-04-01)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica