156558
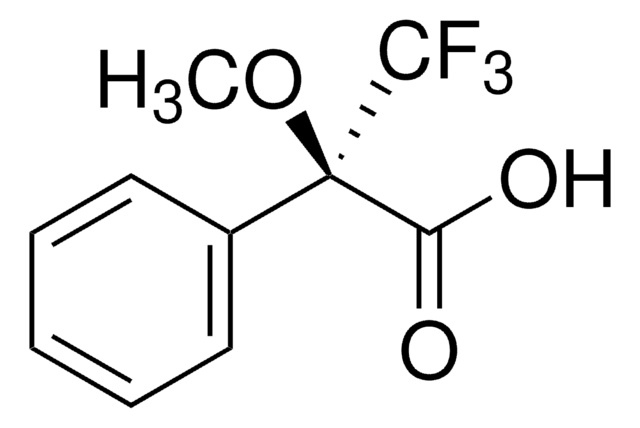
α-Methoxy-α-(trifluoromethyl)phenylacetic acid
97%
Sinônimo(s):
MTPA
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C6H5C(OCH3)(CF3)CO2H
Número CAS:
Peso molecular:
234.17
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
índice de refração
n20/D 1.476 (lit.)
p.e.
95-98 °C/0.05 mmHg (lit.)
pf
41-46 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
COC(C(O)=O)(c1ccccc1)C(F)(F)F
InChI
1S/C10H9F3O3/c1-16-9(8(14)15,10(11,12)13)7-5-3-2-4-6-7/h2-6H,1H3,(H,14,15)
chave InChI
JJYKJUXBWFATTE-UHFFFAOYSA-N
Aplicação
Diastereomeric bis-esters of (±)-trans-7,8-dihydroxy-7,8,9,10-tetrahydrobenzo[α]pyrene with MTPA has been used as a noncarcinogenic precursor to obtain the (+)and (-)-dihydrodiols by chromatographic resolution. It has also been used to develop empirically derived correlation of configuration and nmr chemical shifts for diastereomeric MTPA esters.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nuclear Magnetic Resonance Enantiomer Reagents. Configurational Correlations via Nuclear Magnetic Resonance Chemical Shifts of Diastereomeric Mandelate, 0-Methylmandelate, and α-Methoxy-α-trifluoromethylphenylacetate (MTPA) Esters
Dale JA and Mosher HS
Journal of the American Chemical Society, 95(2), 512-519 (1973)
Yuka Uemura et al.
Chemical & pharmaceutical bulletin, 63(8), 608-616 (2015-08-04)
From the leaves of Meliosma lepidota ssp. squamulata, megastigmane glucosides with spiro-structures and megastigmanes were isolated. Their structures were determined by X-ray crystallographic analyses and spectroscopic investigation. The absolute structures of the megastigmanes were determined by the modified Mosher's method.
W Levin et al.
The Journal of biological chemistry, 255(19), 9067-9074 (1980-10-10)
The (+)- and (-)-enantiomers of benzo[a]pyrene 7,8-oxide are hydrated stereospecifically at C-8 to (-)- and (+)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene, respectively, by rat hepatic epoxide hydrolase. The (-)-enantiomer of benzo[a]pyrene 7,8-oxide is metabolized by microsomal epoxide hydrolase at a rate 3- to 4-fold greater
Dongsheng Du et al.
Fitoterapia, 101, 73-79 (2015-01-07)
Two new germacrane sesquiterpenes, yedoensins A (1) and B (2), together with 8 known ones (3-10) were isolated from the herb of Viola yedoensis. The structures of the new compounds were established by extensive spectroscopic means including 1D ((1)H and
Guangyu Wang et al.
Oncology letters, 10(5), 2835-2841 (2016-01-02)
MicroRNA-181 (miR-181) has been recently demonstrated to participate in the differentiation and development of immune cells, including natural killer cells and B and T lymphocytes, and myeloid linages, including erythroid and megakaryocytic cells. The aberrant expression of miR-181, particularly low
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica