38332
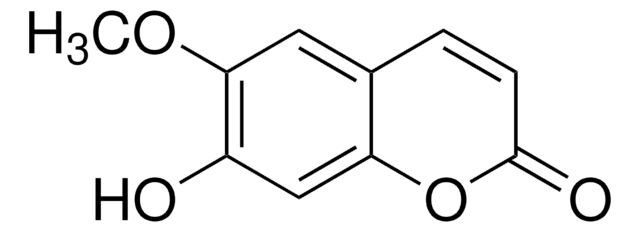
Scopoletin
analytical standard
Synonyme(s) :
6-Methoxyumbelliferone, 6-Methylesculetin, 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one, 7-Hydroxy-6-methoxycoumarin, Buxuletin, Chrysatropic acid, Escopoletin, Esculetin-6-methyl ether, Gelseminic acid, Murrayetin
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Pureté
≥97.0% (HPLC)
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Pf
203-205 °C (lit.)
Application(s)
food and beverages
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
COc1cc2C=CC(=O)Oc2cc1O
InChI
1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3
Clé InChI
RODXRVNMMDRFIK-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Roots, stems, branches, and seeds as well as leaves of Chimonanthus nitens Oliv. by ultra-high performance liquid chromatography/tandem quadrupole-time-of-flight mass spectrometry (UHPLC-QTOF-MS/MS) equipped with modified mass deflect filter or diagnostic product ions/neutral loss filter.
- Canscora decussate (South Indian Shankhpushpi) extracts by high performance thin layer chromatography (HPTLC).
- Morinda tinctoria Roxb. leaf by HPTLC in conjunction with HPLC and gas chromatography with MS.
Actions biochimiques/physiologiques
Conditionnement
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Protocoles
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique