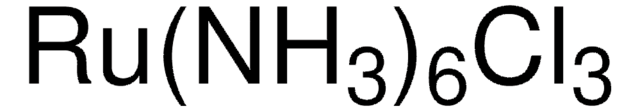450162
Potassium hexachloroiridate(IV)
99.99% trace metals basis
Synonyme(s) :
Iridium potassium chloride
About This Item
Produits recommandés
Pureté
99.99% trace metals basis
Forme
powder
Impuretés
≤150.0 ppm Trace Metal Analysis
Pf
>385 °C (lit.)
Chaîne SMILES
[K+].[K+].Cl[Ir--](Cl)(Cl)(Cl)(Cl)Cl
InChI
1S/6ClH.Ir.2K/h6*1H;;;/q;;;;;;+4;2*+1/p-6
Clé InChI
WEKZJZDULNNSCD-UHFFFAOYSA-H
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Voltammetry at HMT-PMBI-coated glassy carbon electrodes: This study explores the electrochemical properties of potassium hexachloroiridate(IV) and its potential for sensitive detection, which could be valuable in analytical chemistry applications (M Rees et al., 2020).
- Antioxidant Total Capacity Assay: Introduces a novel assaying method for antioxidant capacity using potassium hexachloroiridate(IV), which could have implications in pharmacology and drug discovery (Y Liu et al., 2024).
- Mass Transfer in 3D-printed Electrolyzers: Examines the role of potassium hexachloroiridate(IV) in enhancing mass transfer in electrolytic systems, which is pivotal for chemical engineering and energy applications (SJC Weusten et al., 2021).
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique