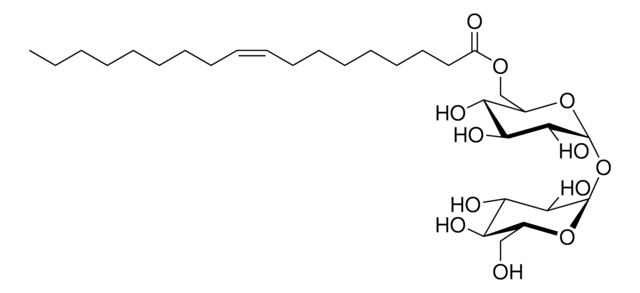
30564
Trehalose-6-hexadecanoat
≥95.0% (GC)
Synonym(e):
α,α-Trehalose-6-palmitat, α-D-Glucopyranosyl-α-D-glucopyranosid-6-hexadecanoat, 6-O-Palmitoyl-α,α-trehalose, Trehalose-6-palmitat
About This Item
Empfohlene Produkte
Assay
≥95.0% (GC)
Form
powder
Mol-Gew.
580.71 g/mol
CMC
0.0061 mM
Lagertemp.
2-8°C
SMILES String
O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@H]2O[C@H](COC(CCCCCCCCCCCCCCC)=O)[C@@H](O)[C@H](O)[C@H]2O
InChI
1S/C28H52O12/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-20(30)37-17-19-22(32)24(34)26(36)28(39-19)40-27-25(35)23(33)21(31)18(16-29)38-27/h18-19,21-29,31-36H,2-17H2,1H3/t18?,19?,21-,22-,23?,24?,25+,26+,27-,28-/m1/s1
InChIKey
VNHFMAKRYDWMAF-DHFWNHDGSA-N
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.