Wichtige Dokumente
158240
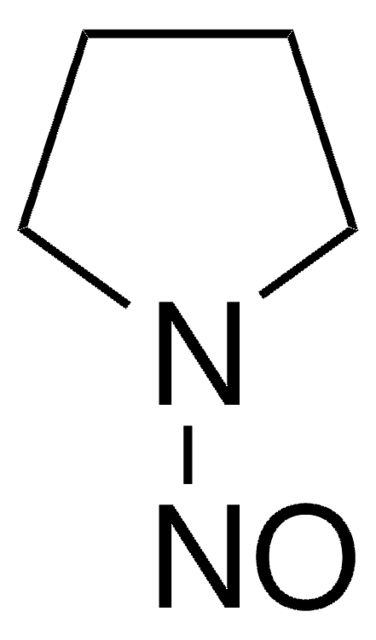
N-Nitroso-pyrrolidin
99%
Synonym(e):
N-Nitrosopyrrolidin, NPYR
About This Item
Empfohlene Produkte
Assay
99%
Brechungsindex
n20/D 1.489 (lit.)
bp
214 °C (lit.)
Dichte
1.085 g/mL at 25 °C (lit.)
SMILES String
O=NN1CCCC1
InChI
1S/C4H8N2O/c7-5-6-3-1-2-4-6/h1-4H2
InChIKey
WNYADZVDBIBLJJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Biochem./physiol. Wirkung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Carc. 2
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
208.4 °F - closed cup
Flammpunkt (°C)
98 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.