92330
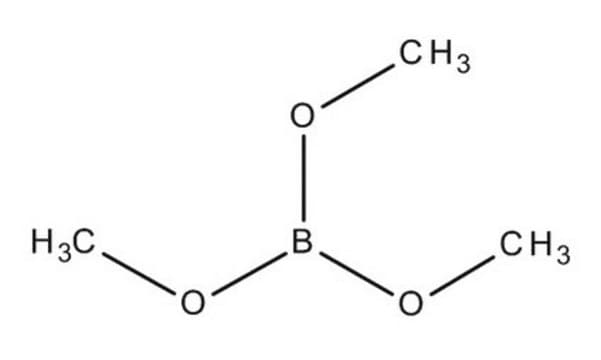
Trimethylborat
purum, ≥99.0% (GC)
Synonym(e):
Borsäure-trimethylester, Methylborat
About This Item
Empfohlene Produkte
Dampfdichte
3.59 (vs air)
Qualitätsniveau
Qualität
purum
Assay
≥99.0% (GC)
Form
liquid
Brechungsindex
n20/D 1.346 (lit.)
n20/D 1.358
bp
68-69 °C (lit.)
mp (Schmelzpunkt)
−34 °C (lit.)
Dichte
0.932 g/mL at 20 °C (lit.)
SMILES String
COB(OC)OC
InChI
1S/C3H9BO3/c1-5-4(6-2)7-3/h1-3H3
InChIKey
WRECIMRULFAWHA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- For the synthesis of luminogens having triphenylamine core and tetraphenylethene peripheral moieties.
- For the synthesis of ammonia borane and trialkylamine boranes.
- In the reduction of carboxylic acids in the presence of borane-methyl sulfide.
- As a reagent to crosslink phopshate complexes.
- As a source of boron to synthesize boron nitride nanotubes by chemical vapor deposition (CVD) method.
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 1B - STOT SE 1
Zielorgane
Eyes
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 1
Flammpunkt (°F)
12.2 °F - (own results)
Flammpunkt (°C)
-11 °C - (own results)
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.