Wichtige Dokumente
931853
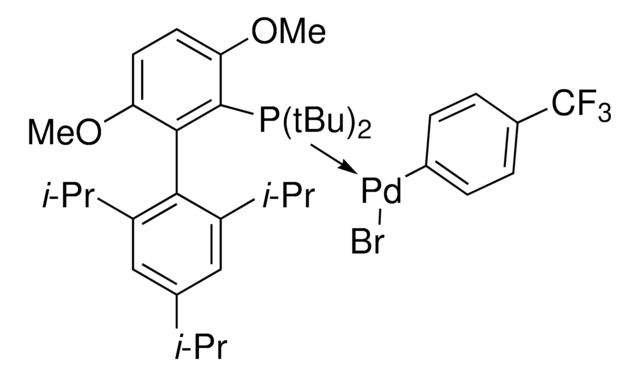
SPhos Pd G6 acylation
≥95%
Synonym(e):
(SPhos)Pd(4-CH2CH2CONHSPh)Br
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
powder
Eignung der Reaktion
reagent type: catalyst
reaction type: Cross Couplings
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Grünere Alternativprodukt-Kategorie
Allgemeine Beschreibung
Anwendung
Learn more about G6 Buchwald precatalysts
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Buchwald G6 precatalysts are oxidative addition complexes that are thermally, air, and moisture stable. The catalyst activation doesn’t require a base and generates innocuous byproducts.
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.