91779
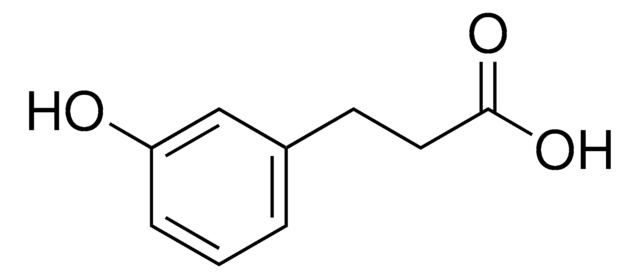
3-(3-Hydroxyphenyl)propionic acid
analytical standard
Sinónimos:
3-(3-Hydroxyphenyl)propanoic acid, NSC 33135, NSC 39468
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H10O3
Número de CAS:
Peso molecular:
166.17
Beilstein:
1947445
Número CE:
Número MDL:
Código UNSPSC:
12352200
ID de la sustancia en PubChem:
NACRES:
NA.24
Productos recomendados
grado
analytical standard
Nivel de calidad
Ensayo
≥98.0% (HPLC)
caducidad
limited shelf life, expiry date on the label
aplicaciones
clinical testing
Formato
neat
cadena SMILES
OC(CCC1=CC=CC(O)=C1)=O
InChI
1S/C9H10O3/c10-8-3-1-2-7(6-8)4-5-9(11)12/h1-3,6,10H,4-5H2,(H,11,12)
Clave InChI
QVWAEZJXDYOKEH-UHFFFAOYSA-N
Acciones bioquímicas o fisiológicas
3-(3-Hydroxyphenyl)propanoic acid is one of the major metabolites of ingested caffeic acid and of the phenolic degradation products of proanthocyanidins (the most abundant polyphenol present in chocolate) by the microflora in the colon. 3-(3-Hydroxyphenyl)propanoic acid is suspected to have antioxidants properties and is actively absorbed by the monocarboxylic acid transporter (MCT) in intestinal Caco-2 cell monolayers.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Jin-Ran Chen et al.
Communications biology, 4(1), 53-53 (2021-01-10)
The G protein-coupled receptor 109 A (GPR109A) is robustly expressed in osteoclastic precursor macrophages. Previous studies suggested that GPR109A mediates effects of diet-derived phenolic acids such as hippuric acid (HA) and 3-(3-hydroxyphenyl) propionic acid (3-3-PPA) on promoting bone formation. However, the
Letizia Bresciani et al.
Food research international (Ottawa, Ont.), 141, 110137-110137 (2021-03-02)
Cranberries (Vaccinium macrocarpon) represent an important source of anthocyanins, flavan-3-ols and flavonols. This study aimed at investigating in vitro the human microbial metabolism of (poly)phenols, principally flavan-3-ols, of unformulated- and phytosome-formulated cranberry extracts. After powder characterization, a 24-h fermentation with
Eileen Carry et al.
Journal of pharmaceutical and biomedical analysis, 159, 374-383 (2018-07-23)
Grape-derived products contain a wide array of bioactive phenolic compounds which are of significant interest to consumers and researchers for their multiple health benefits. The majority of bioavailable grape polyphenols, including the most abundant flavan-3-ols, i.e. (+)-catechin and (-)-epicatechin, undergo
Veronika Pilařová et al.
Talanta, 185, 71-79 (2018-05-16)
Fast, selective, and sensitive ultra-high performance liquid chromatography method with tandem mass spectrometry detection for the determination of quercetin and its metabolites with various physico-chemical properties such as molecular weight, lipophilicity, and acid-base properties has been developed. These compounds included
Yutaka Konishi et al.
Journal of agricultural and food chemistry, 52(21), 6418-6424 (2004-10-14)
It was previously reported that m-coumaric acid, m-hydroxyphenylpropionic acid (mHPP), and 3,4-dihydroxyphenylpropionic acid (DHPP) are major metabolites of ingested caffeic acid formed by gut microflora and would be transported by the monocarboxylic acid transporter (MCT). We have directly measured their
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico