912719
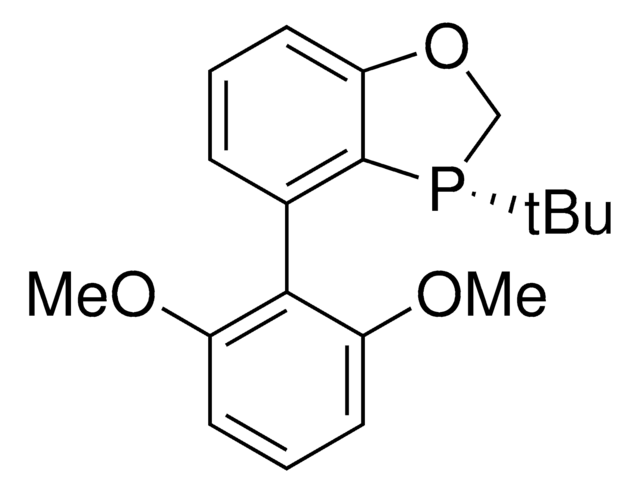
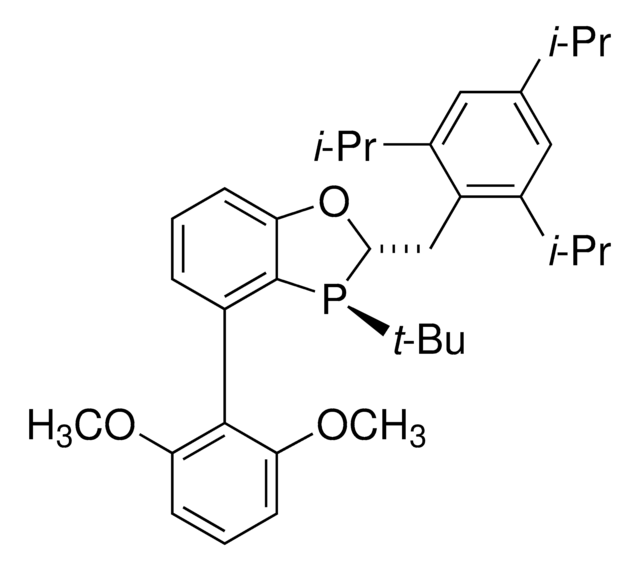
(S)-BIDIME
≥97%
Synonym(s):
(S)-3-(tert-butyl)-4-(2,6-dimethoxyphenyl)-2,3-dihydrobenzo[d][1,3]oxaphosphole
Sign Into View Organizational & Contract Pricing
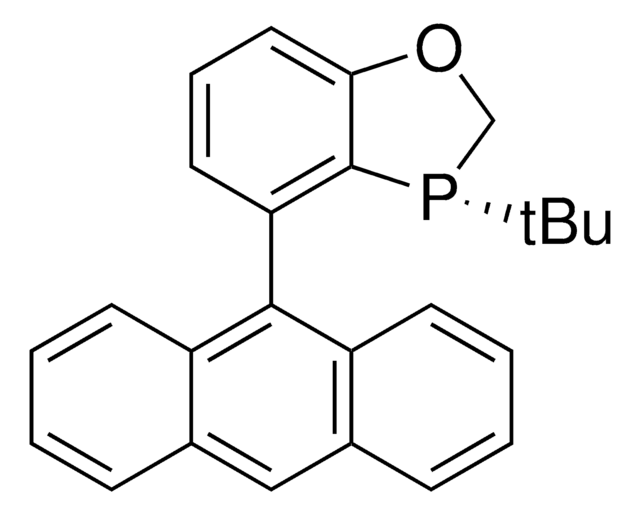
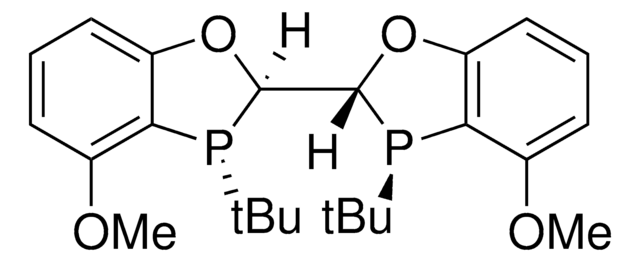
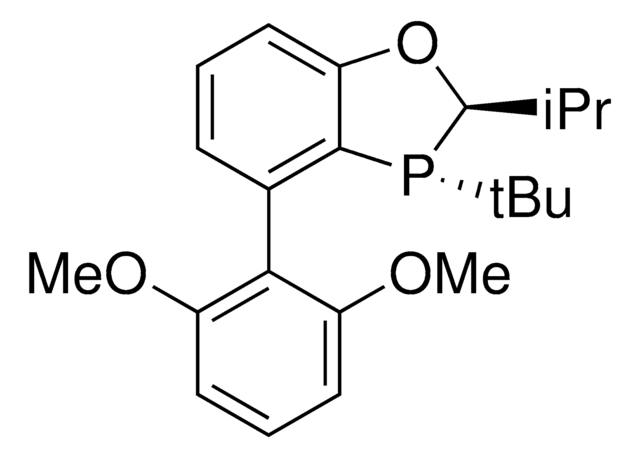
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C19H23O3P
CAS Number:
Molecular Weight:
330.36
UNSPSC Code:
12352200
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
powder
optical purity
ee: ≥99% (HPLC)
reaction suitability
reagent type: ligand
functional group
phosphine
Application
(S)-BIDIME is a P-chiral monophosphorus ligand used for the transition metal-catalyzed asymmetric Suzuki-Miyaura and hydroboration reactions.
Legal Information
Sold in collaboration with Zejun Pharmaceuticals
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wenzhen Fu et al.
Angewandte Chemie (International ed. in English), 54(8), 2520-2524 (2015-01-20)
The first asymmetric nickel-catalyzed intramolecular reductive cyclization of alkynones is reported. A P-chiral monophosphine and triethylsilane were used as the ligand and the reducing reagent, respectively, to form a series of tertiary allylic alcohols bearing furan/pyran rings in excellent yields
Ruofei Cheng et al.
Journal of the American Chemical Society, 140(13), 4508-4511 (2018-03-27)
Carborane cage chirality is an outstanding issue of great interest as the icosahedral carboranes have wide applications in medicinal and materials chemistry. The synthesis of optically active carborane derivatives, whose chirality is associated with the substitution patterns on the polyhedron
Naifu Hu et al.
Journal of the American Chemical Society, 137(21), 6746-6749 (2015-05-06)
The rhodium-catalyzed asymmetric hydroboration of α-arylenamides with BI-DIME as the chiral ligand and (Bpin)2 as the reagent yields for the first time a series of α-amino tertiary boronic esters in good yields and excellent enantioselectivities (up to 99% ee).
Naifu Hu et al.
Angewandte Chemie (International ed. in English), 55(16), 5044-5048 (2016-03-19)
A highly enantioselective alkene aryloxyarylation led to the high-yielding formation of a series of 1,4-benzodioxanes, 1,4-benzooxazines, and chromans containing quaternary stereocenters with excellent enantioselectivity. The sterically bulky and conformationally well defined chiral monophosphorus ligand L4 or L5 was responsible for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service