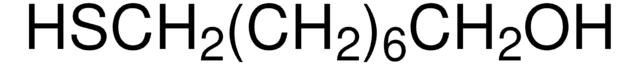698768
9-Mercapto-1-nonanol
96%
Synonym(s):
9-Mercapto-nonan-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H20OS
CAS Number:
Molecular Weight:
176.32
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.483
density
0.939 g/mL at 25 °C
SMILES string
OCCCCCCCCCS
InChI
1S/C9H20OS/c10-8-6-4-2-1-3-5-7-9-11/h10-11H,1-9H2
InChI key
FXFJFNVBVKPAPL-UHFFFAOYSA-N
General description
9-Mercapto-1-nonanol (9-MNL) is an alkanethiol that forms a self-assembled monolayer (SAM) on a variety of substrates. It facilitates the immobilization of the surface atoms.
Application
9-MNL can be used in the surface modification of gold electrodes for the fabrication of biosensors for biomedical applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Peptide-based electrochemical biosensor for amyloid beta 1-42 soluble oligomer assay
Li H, et al.
Talanta, 93, 358-363 (2012)
An electrochemical peptide-based biosensing platform for HIV detection
Gerasimov JY and Lai RY
Chemical Communications (Cambridge, England), 46(3), 395-397 (2010)
Label-free capacitive DNA sensor using immobilized pyrrolidinyl PNA probe: effect of the length and terminating head group of the blocking thiols
Thipmanee O, et al.
Biosensors And Bioelectronics, 38(1), 430-435 (2012)
Ivan V Smolyaninov et al.
Bioorganic chemistry, 89, 103003-103003 (2019-05-28)
A number of asymmetrical thioethers based on 3,5-di-tert-butylcatechol containing sulfur atom bonding with physiologically active groups in the sixth position of aromatic ring have been synthesized and the electrochemical properties, antioxidant, cryoprotective activities of new thioethers have been evaluated. Cyclic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service