448729
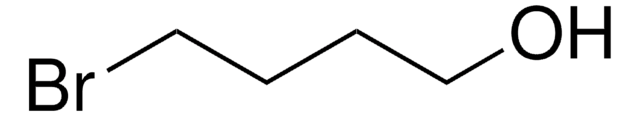
9-Bromo-1-nonanol
95%
Synonym(s):
Nonamethylene bromohydrin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)9OH
CAS Number:
Molecular Weight:
223.15
Beilstein:
1737525
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
125-126 °C/2 mmHg (lit.)
mp
33-35 °C (lit.)
functional group
bromo
hydroxyl
SMILES string
OCCCCCCCCCBr
InChI
1S/C9H19BrO/c10-8-6-4-2-1-3-5-7-9-11/h11H,1-9H2
InChI key
USJDOLXCPFASNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of the diacetylenic phospholipids 1, 2-(10', 12'-heptadecadiynoyl)-sn-glycero-3-phophatidylcholine and 1, 2-(4', 6'-tricosadiynoyl)-sn-glycero-3-phophatidylcholine.
Hennies PT, et al.
Journal of the Brazilian Chemical Society, 12(1), 64-72 (2001)
In vitro enzymatic oxidation of a fluorine-tagged sulfido substrate analogue: a 19F NMR investigation.
Tremblay AE, et al.
Magnetic Resonance in Chemistry, 44(6), 629-632 (2006)
Néstor M Carballeira et al.
Chemistry and physics of lipids, 145(1), 37-44 (2006-11-28)
The first total syntheses for the (Z)-15-methyl-10-hexadecenoic acid and the (Z)-13-methyl-8-tetradecenoic acid were accomplished in seven steps and in 31-32% overall yields. The (trimethylsilyl)acetylene was the key reagent in both syntheses. It is proposed that the best synthetic strategy towards
Sequential alkynylation of ω-bromoalkyl triflates: facile access to unsymmetrical non-conjugated diynes including precursors to diene.
Armstrong-Chong RJ, et al.
Tetrahedron, 60(45), 10239-10244 (2004)
Highly ion conductive flexible films composed of network polymers based on polymerizable ionic liquids.
Washiro S, et al.
Polymer, 45(5), 1577-1582 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![Poly[4,4′-methylenebis(phenyl isocyanate)-alt-1,4-butanediol/di(propylene glycol)/polycaprolactone] pellets, MDI-polyester/polyether polyurethane.](/deepweb/assets/sigmaaldrich/product/structures/661/697/b23c24ce-15fb-4eae-a30f-786921d4c91e/640/b23c24ce-15fb-4eae-a30f-786921d4c91e.png)