55453
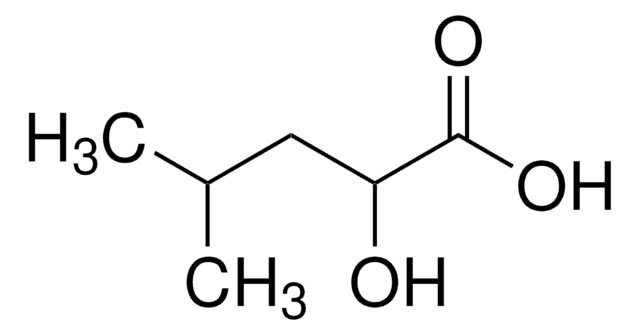
β-Hydroxyisovaleric acid
≥95.0% (T)
Synonym(s):
3-Hydroxy-3-methylbutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10O3
CAS Number:
Molecular Weight:
118.13
Beilstein:
1743952
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (T)
refractive index
n20/D 1.4415 (lit.)
bp
88 °C/1 mmHg (lit.)
mp
−80 °C (lit.)
density
0.938 g/mL at 25 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
CC(C)(O)CC(O)=O
InChI
1S/C5H10O3/c1-5(2,8)3-4(6)7/h8H,3H2,1-2H3,(H,6,7)
InChI key
AXFYFNCPONWUHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
β-Hydroxyisovaleric acid can be used as as a precursor for steroid alcohols.
It is widely used in the synthesis of the anthrax tetrasaccharide and its analogs as anthrax vaccine candidates. β-hydroxyisovaleric acid can also be used in the synthesis of β-lactones by intramolecular dehydration condensation using 2-methyl-6-nitrobenzoic anhydride (MNBA).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of antigen Bacillus anthracis tetrasaccharide?creation of an anthrax vaccine candidate.
Werz, Daniel B and Seeberger, Peter H
Angewandte Chemie (International Edition in English), 44(39), 6315-6318 (2005)
De novo asymmetric synthesis of anthrax tetrasaccharide and related tetrasaccharide.
Guo, Haibing and O?Doherty, George A
The Journal of Organic Chemistry, 73(14), 5211-5220 (2008)
Carlos Hermano da Justa Pinheiro et al.
European journal of applied physiology, 112(7), 2531-2537 (2011-11-15)
Beta-hydroxy-beta-methylbutyrate (HMB) is a metabolite derived from leucine. The anti-catabolic effect of HMB is well documented but its effect upon skeletal muscle strength and fatigue is still uncertain. In the present study, male Wistar rats were supplemented with HMB (320
Shawn Portal et al.
European journal of applied physiology, 111(9), 2261-2269 (2011-02-18)
The use of ergogenic nutritional supplements is becoming inseparable from competitive sports. β-Hydroxy-β-Methylbutyric acid (HMB) has recently been suggested to promote fat-free mass (FFM) and strength gains during resistance training in adults. In this prospective randomized, double-blind, placebo-controlled study, we
C Flummer et al.
Journal of animal science, 90 Suppl 4, 372-374 (2013-02-13)
This trial was conducted to investigate whether β-hydroxy β-methyl butyrate (HMB) supplementation during late gestation and throughout lactation would influence colostrum and milk production of sows and neonatal piglet survival (0 to 24 h). Control sows (CON; n = 8)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[(3R)-3-Hydroxydodecanoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/307/615/dadd9469-b690-4f3b-8106-1471f02b2385/640/dadd9469-b690-4f3b-8106-1471f02b2385.png)

![[(3R)-3-Hydroxy-cis-octadec-9-enoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/216/153/a7c10b3a-07ce-4b6e-b7cd-b1a180a75a2d/640/a7c10b3a-07ce-4b6e-b7cd-b1a180a75a2d.png)