389439
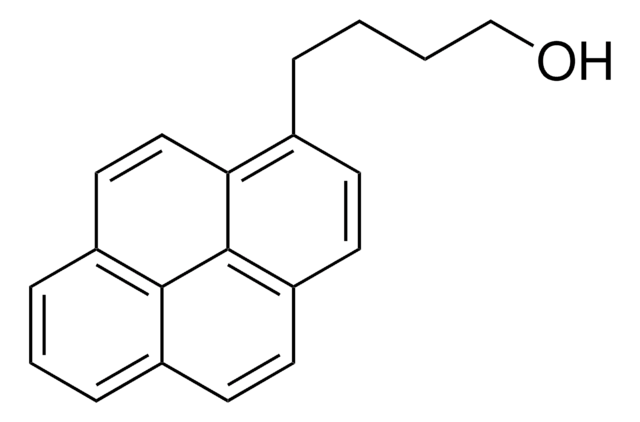
1-Pyrenemethanol
98%
Synonym(s):
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C17H12O
CAS Number:
Molecular Weight:
232.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
123-126 °C (lit.)
functional group
hydroxyl
SMILES string
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
InChI key
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Application
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Glatt et al.
Chemico-biological interactions, 109(1-3), 195-219 (1998-05-05)
Sulfation is a common final step in the biotransformation of xenobiotics and is traditionally associated with inactivation. However, the sulfate group is electron-withdrawing and may be cleaved off heterolytically in some molecules leading to electrophilic cations which may form adducts
Walter Meinl et al.
Pharmacogenetics, 12(9), 677-689 (2002-12-05)
Various enzymatically formed sulfuric acid esters are chemically reactive and mutagenic. This metabolic activation pathway is not detected in standard in-vitro mutagenicity test systems. We describe the construction of Salmonella typhimurium TA1538-derived strains expressing alloenzymes *1, *2, *3, *5, *6
Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst.
Ji H, et al.
Tetrahedron Letters, 43(40), 7179-7183 (2002)
H Glatt et al.
Chemico-biological interactions, 92(1-3), 305-319 (1994-06-01)
1-Hydroxymethylpyrene (HMP) is activated to a potent mutagen, detectable in Salmonella typhimurium, in the presence of hepatic cytosol, cofactor for sulfotransferases, and chloride anions. The number of induced mutations is linear to the amount of cytosol used over a wide
Y J Surh et al.
Carcinogenesis, 11(9), 1451-1460 (1990-09-01)
Our previous studies on 7-hydroxymethyl-12-methylbenz[a]anthracene and 6-hydroxymethylbenzo[a]pyrene showed that cytosolic sulfotransferase activity plays a major role in the formation of hepatic benzylic DNA and RNA adducts by these carcinogens in rats. In the present study, we found similar sulfotransferase activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service