198242
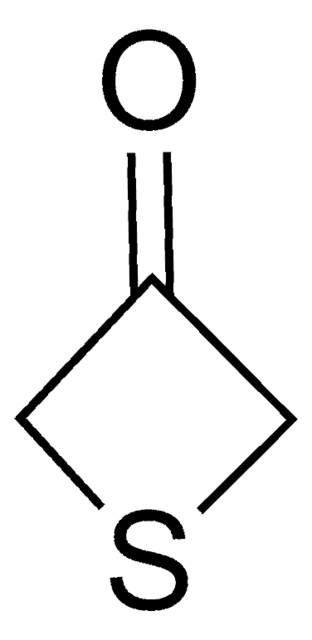
Tetrahydro-4H-pyran-4-one
99%
Synonym(s):
4-Oxotetrahydropyran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein:
106463
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.452 (lit.)
bp
166-166.5 °C (lit.)
density
1.084 g/mL at 25 °C (lit.)
functional group
ether
ketone
SMILES string
O=C1CCOCC1
InChI
1S/C5H8O2/c6-5-1-3-7-4-2-5/h1-4H2
InChI key
JMJRYTGVHCAYCT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
131.0 °F - closed cup
Flash Point(C)
55 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R K Anderson et al.
The Journal of antibiotics, 46(2), 331-342 (1993-02-01)
Syntheses are described for penicillins (4b approximately 4i, 5a and 5b) which possess a 6 beta-(2-heteroaryl-3-substituted)-propenamido side-chain of fixed geometry. In vitro results for these compounds against a range of Gram-positive and Gram-negative bacteria showed in most cases good stability
Bryan Ringstrand et al.
Beilstein journal of organic chemistry, 7, 386-393 (2011-04-23)
The methodology to prepare 3-substituted 1,5-dibromopentanes I and their immediate precursors, which include 3-substituted 1,5-pentanediols VII or 4-substituted tetrahydropyrans VIII, is surveyed. Such dibromides I are important intermediates in the preparation of liquid crystalline derivatives containing 6-membered heterocyclic rings. Four
Tetrahedron Letters, 33, 7701-7701 (1992)
Synthesis, 672-672 (1994)
Kun Huang et al.
The Journal of organic chemistry, 71(21), 8320-8323 (2006-10-10)
As the first example for the synthesis of optically active alpha-hydroxyaldehydes and alpha-hydroxyketones in ionic liquids, we applied RTILs into L-proline catalyzed direct enantioselective alpha-aminoxylation of both aldehydes and ketones successfully. This protocol features a number of advantages, such as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic dianhydride 99%](/deepweb/assets/sigmaaldrich/product/structures/418/038/9edd3533-0f32-442c-8a1f-4e154e65c3b5/640/9edd3533-0f32-442c-8a1f-4e154e65c3b5.png)