All Photos(1)
About This Item
Empirical Formula (Hill Notation):
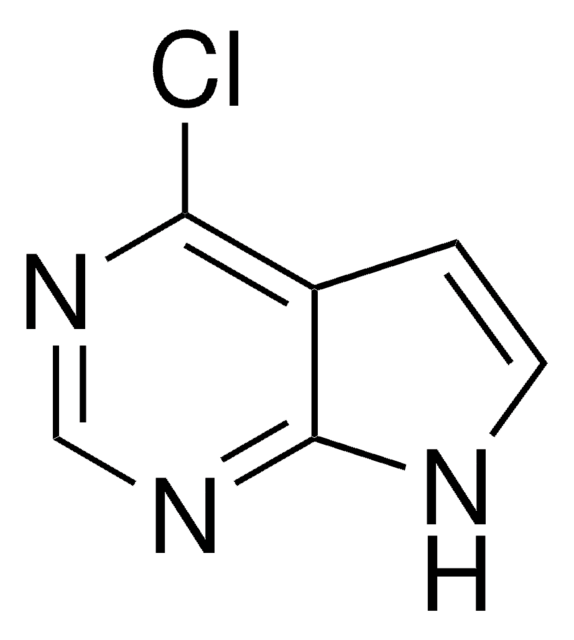
C5H3ClN4
CAS Number:
Molecular Weight:
154.56
Beilstein:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
mp
>300 °C (dec.) (lit.)
solubility
DMF: soluble 5%, clear, colorless to yellow
functional group
chloro
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-O-acetyl-D-xylal has been investigated.
Application
6-Chloropurine has been used in the preparation of 9-alkylpurines via alkylation with various substituted alkyl halides in DMSO. It was also used in the preparation of 6-succinoaminopurine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Shi Bai et al.
Magnetic resonance in chemistry : MRC, 48(1), 61-67 (2009-11-26)
The (15)N and (13)C chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleoside derivatives in deuterated chloroform were measured. The (15)N chemical shifts were determined by the (1)H-(15)N HMBC method, and complete (15)N chemical-shift assignments were made with the aid
Hai-Ming Guo et al.
The Journal of organic chemistry, 75(17), 6016-6018 (2010-08-12)
Highly functionalized C6-aryl-substituted purine analogues were synthesized through direct arylation of 6-chloropurine with aromatics promoted by anhydrous AlCl(3) in a single step. The reactions, which were conducted using a 3-fold excess of AlCl(3) in refluxing 1,2-dichloroethane, gave moderate to excellent
Silvia Zimdars et al.
Organic letters, 13(4), 792-795 (2011-01-21)
Starting from an appropriate 6-chloro-2-TMS-purine derivative, a regioselective functionalization of the purine scaffold was achieved successively at positions 8, 6, and 2 via zinc and magnesium intermediates which were generated either by a direct zincation with TMPZnCl·LiCl or by an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service