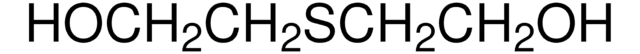88561
2,2′-Thiodiethanol
purum, ≥95.0% (GC)
Synonym(s):
2,2′-Thiobis(ethanol), Bis(2-hydroxyethyl) sulfide, Thiodiethylene glycol, Thiodiglycol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
S(CH2CH2OH)2
CAS Number:
Molecular Weight:
122.19
Beilstein:
1236325
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥95.0% (GC)
form
liquid
refractive index
n20/D 1.521
n20/D 1.5215 (lit.)
bp
164-166 °C/20 mmHg (lit.)
mp
−16 °C (lit.)
density
1.221 g/mL at 25 °C (lit.)
SMILES string
OCCSCCO
InChI
1S/C4H10O2S/c5-1-3-7-4-2-6/h5-6H,1-4H2
InChI key
YODZTKMDCQEPHD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,2′-Thiodiethanol is used in the preparation of a wide range of gold complexes, which include Au(I) based catalysts such as chloro(triphenylphosphine)gold(I) [Au(PPh3)Cl], chloro(dimethyl sulfide)gold(I) [Au(SMe2)Cl] and chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]gold(I) [IPrAuCl]. It can also be used to build calixarene frameworks.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gold (I)?Catalyzed Intermolecular Cyclopropanation of Enynes with Alkenes: Trapping of Two Different Gold Carbenes.
Lopez S, et al.
Angewandte Chemie (International Edition in English), 118(36), 6175-6178 (2006)
Stereo-and regioselective gold-catalyzed hydroamination of internal alkynes with dialkylamines.
Hesp K D, et al.
Journal of the American Chemical Society, 132(51), 18026-18029 (2010)
Binding and extraction of alkali and alkaline earth metals by nano-baskets of calix [4] arene-1, 2-crown-3.
Mokhtari B and Pourabdollah K
Journal of Inclusion Phenomena and Macrocyclic Chemistry, 73(1-4), 269-277 (2012)
A general synthesis for gold (I) complexes.
Al?Sa'Ady A K, et al.
Inorg. Synth., 23, 191-194 (1985)
Networking of calixcrowns: From heteronuclear endo/exocyclic coordination polymers to a photoluminescence switch.
Lee J Y, et al.
Journal of the American Chemical Society, 130(42), 13838-13839 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service