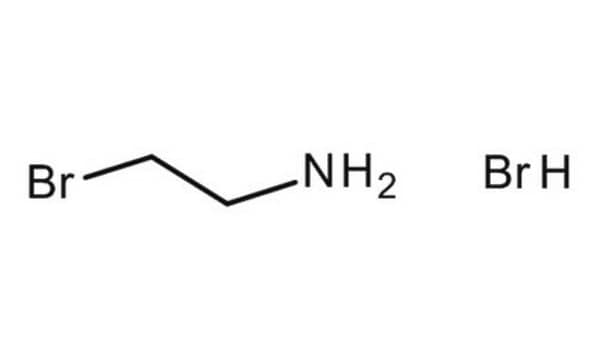
C40200
2-Chloroethylamine hydrochloride
99%
Synonym(s):
2-Aminoethyl chloride hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
ClCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
115.99
Beilstein:
3590304
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
140-150 °C (lit.)
SMILES string
Cl.NCCCl
InChI
1S/C2H6ClN.ClH/c3-1-2-4;/h1-2,4H2;1H
InChI key
ONRREFWJTRBDRA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Chloroethylamine hydrochloride is used:
- in the preparation of novel Schiff base ligands, that can also be used to remove metal ions such as Co (II) and Ni (II) from contaminated water when immobilized on silica gels)
- as a derivatizing reagent
Derivatizing reagent for amino acids, dipeptides, and nucleotides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Muta. 2 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Sosnovsky et al.
Journal of pharmaceutical sciences, 82(1), 1-10 (1993-01-01)
A series of L,L- (42, 44, 46, and 60) and D,D- (43, 45, 47, and 61) dipeptide derivatives composed of phenylglycine, phenylalanine, homophenylalanine, and valine and containing a 2-chloroethylamino group at the C-terminus and an N'-(2-chloroethyl)-N'-nitroso-aminocarbonyl group at the N-terminus
A Chur et al.
Nucleic acids research, 21(22), 5179-5183 (1993-11-11)
The condensation of 5'-O-protected 3'-O-(2-aminoethyl)thymidine with 1,2-dideoxy-1-thyminyl-beta-D-erythro-pentofuranuronic acid gives a T*T dimer with * representing a 3'-OCH2CH2NHC(O)-4' linkage connecting the two pentofuranosyl moieties. The incorporation of this dimer in oligonucleotide sequences show only moderately lowered Tm values when hybridized with
Norlaily Ahmad et al.
Biomacromolecules, 20(7), 2506-2514 (2019-06-28)
Inflammatory conditions are frequently accompanied by increased levels of active proteases, and there is rising interest in methods for their detection to monitor inflammation in a point of care setting. In this work, new sensor materials for disposable single-step protease
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service