H53704
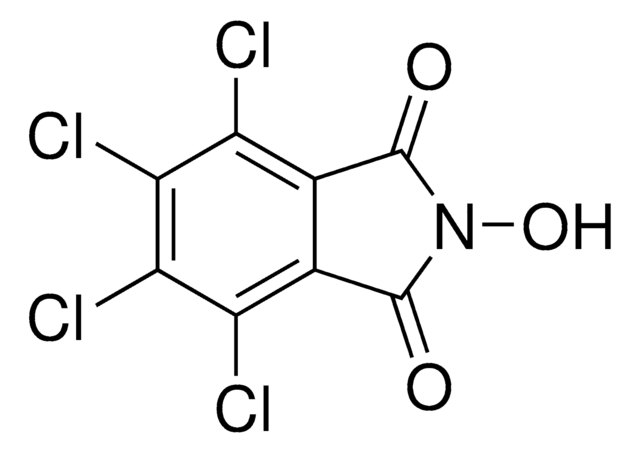
N-Hydroxyphthalimide
97%, for peptide synthesis
Synonym(s):
2-Hydroxy-1H-isoindole-1,3(2H)-dione, 2-Hydroxyisoindole-1,3-dione, 2-Hydroxyphthalimide
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C8H5NO3
CAS Number:
Molecular Weight:
163.13
Beilstein:
131208
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
N-Hydroxyphthalimide, 97%
Quality Level
Assay
97%
form
crystalline
reaction suitability
reaction type: Addition Reactions
mp
233 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
ON1C(=O)c2ccccc2C1=O
InChI
1S/C8H5NO3/c10-7-5-3-1-2-4-6(5)8(11)9(7)12/h1-4,12H
InChI key
CFMZSMGAMPBRBE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
For preparation of amine nucleosides for coupling into non-anionic, anti-sense oligonucleosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 33, 2645-2645 (1992)
Francesca D'Acunzo et al.
European journal of biochemistry, 269(21), 5330-5335 (2002-10-24)
To investigate how solubility and steric issues affect the laccase-catalysed oxidation of phenols, a series of oligomeric polyphenol compounds, having increasing size and decreasing solubility in water, was incubated with laccase. The extent of substrate conversion, and the nature of
Riyuan Lin et al.
Organic letters, 14(16), 4158-4161 (2012-08-03)
A metal-free N-hydroxyphthalimide (NHPI) catalyzed aerobic oxidative cleavage of olefins has been developed. Molecular oxygen is used as the oxidant and reagent for this oxygenation reaction. This methodology has prevented the use of toxic metals or overstoichiometric amounts of traditional
Yuuki Amaoka et al.
The Journal of organic chemistry, 77(22), 9959-9969 (2012-11-02)
A direct conversion of C(sp(3))-H bonds to C(sp(3))-N bonds has been achieved by utilizing catalytic N-hydroxyphthalimide (NHPI) and stoichiometric dialkyl azodicarboxylate. NHPI functions as a precursor of the electron-deficient phthalimide N-oxyl radical (PINO) to abstract hydrogens, and dialkyl azodicarboxylate acts
Application of a dual linker with a reference cleavage site to discover a new reaction between amines and N-hydroxyphthalimide.
Viktor Krchnák et al.
Journal of combinatorial chemistry, 7(4), 523-525 (2005-07-12)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)