471933
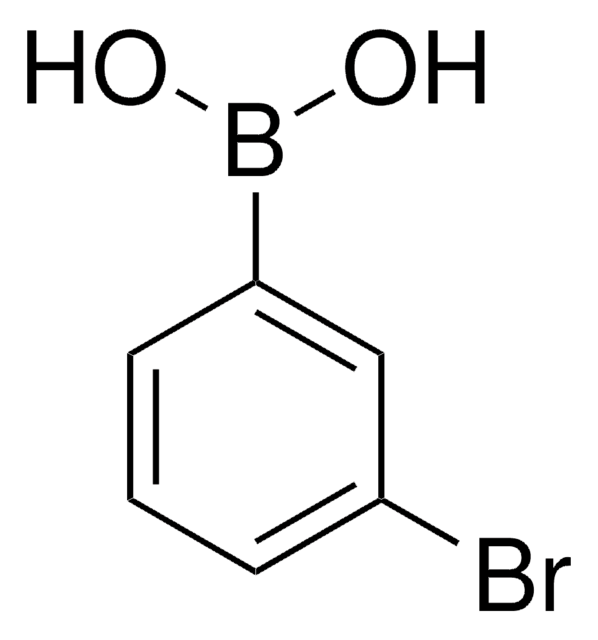
4-Iodophenylboronic acid
≥95.0%
Synonym(s):
B-(4-iodophenyl)-boronic acid, p-Iodophenylboronic acid, p-iodo-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
IC6H4B(OH)2
CAS Number:
Molecular Weight:
247.83
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
326-330 °C (lit.)
functional group
iodo
SMILES string
OB(O)c1ccc(I)cc1
InChI
1S/C6H6BIO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
PELJYVULHLKXFF-UHFFFAOYSA-N
Related Categories
Application
Reagent used for
Reagent used in Preparation of
- Copper-mediated ligandless aerobic fluoroalkylation
- Palladium-catalyzed aerobic oxidative cross-coupling reactions
- Recyclable magnetic-nanoparticle-supported palladium catalyst for the Suzuki coupling reactions
- Oxidative hydroxylation using a copper (Cu) catalyst
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-coupling
- Homocoupling using gold salts as a catalyst
- Ruthenium (Ru)-catalyzed cross-coupling
- CuI-catalyzed Suzuki coupling reactions
- Palladium-catalyzed domino Heck-Mizoroki/Suzuki-Miyaura reactions
- Manganese triacetate-mediated radical additions of arylboronic acids to alkenes
Reagent used in Preparation of
- Pleuromutilin derivatives for ribosomal binding and antibacterial activity via "Click Chemistry"
- Liquid crystalline polyacetylene derivatives
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
CuI-catalyzed Suzuki coupling reaction of organoboronic acids with alkynyl bromides
S. Wang, et al.,
Tetrahedron, 67, 4800-4806 (2011)
B M Kinsey et al.
Nuclear medicine and biology, 20(1), 13-22 (1993-01-01)
Three iodinated phenylboronic acids have been synthesized: 4-iodophenylboronic acid (2a), 3-(4-iodobenzenesulfonamido)phenylboronic acid (5a) and 3-(5-dimethylamino-6-iodo-1-naphthalenesulfonamido)phenylboronic acid (6a). The corresponding no-carrier-added 125I derivatives 2b, 5b and 6b have been prepared in good yield by selective displacement of the tributylstannyl group. Compound
Kiyofumi Inamoto et al.
Chemical communications (Cambridge, England), 47(42), 11775-11777 (2011-10-01)
Copper-catalysed oxidative hydroxylation of arylboronic acids was accomplished in water containing an amphiphilic surfactant, providing facile access to phenol derivatives.
Disubstituted Liquid Crystalline Polyacetylene Derivatives That Exhibit Linearly Polarized Blue and Green Emissions
San Jose, B. A.; et al.
Macromolecules, 44, 6288-6302 (2011)
Alison M Berezuk et al.
Scientific reports, 8(1), 12933-12933 (2018-08-30)
In Escherichia coli, formation of new cells is mediated by the elongasome and divisome that govern cell elongation and septation, respectively. Proper transition between these events is essential to ensure viable progeny are produced; however, the components of each complex
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service