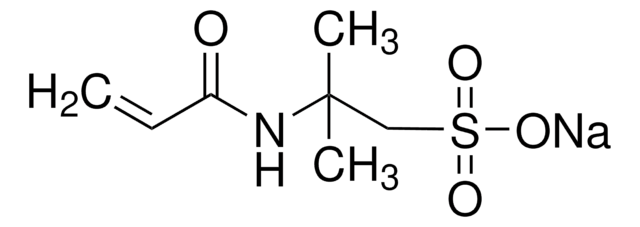
278424
Poly(vinylsulfonic acid, sodium salt) solution
30-40 wt. % in H2O, technical grade
Synonym(s):
PVSA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H3NaO3S)n
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
Quality Level
form
liquid
concentration
30-40 wt. % in H2O
refractive index
n20/D 1.389
density
1.267 g/mL at 25 °C
SMILES string
[Na]OS(=O)(=O)C=C
InChI
1S/C2H4O3S.Na/c1-2-6(3,4)5;/h2H,1H2,(H,3,4,5);/q;+1/p-1
InChI key
BWYYYTVSBPRQCN-UHFFFAOYSA-M
Application
Poly(vinylsulfonicacid, sodium salt) (PVSA) can be used:
- In the preparation of superabsorbent semi-IPN (interpenetrating polymer network) hydrogel.
- As a solid electrolyte for proton conduction in the fabrication of an all-solid-supercapacitor.
- As a crystallization controlling agent in the preparation of high-quality crystals of porous coordination polymers(CP). PVSA regulates not only the size and structure of the crystals but also their preference orientation, resulting in CP channel alignment in the bulk powdery state.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shaoling Cheng et al.
Journal of chromatography. A, 1108(1), 43-49 (2006-01-31)
The goal of this work was to investigate the synergistic effect between the electrostatic and hydrophobic interactions upon the uptake of organic ions with hydrophobic moieties by ion-exchange resins with hydrophobic matrixes. The uptake of neutral amino acids by a
Fatma Arslan et al.
Sensors (Basel, Switzerland), 11(8), 8152-8163 (2011-12-14)
In this study, a novel amperometric glucose biosensor with immobilization of glucose oxidase on electrochemically polymerized polyaniline-polyvinylsulphonate (Pani-Pvs) films has been accomplished via the entrapment technique. Electropolymerization of aniline on the Pt surface of the Pt electrode was carried out
Srivatsan Kidambi et al.
Journal of the American Chemical Society, 126(50), 16286-16287 (2004-12-17)
The development of new methods for fabricating thin films that provide precise control of the three-dimensional topography and cell adhesion could lead to significant advances in the fields of tissue engineering and biosensors. This Communication describes the successful attachment and
Ozlem Colak et al.
Artificial cells, blood substitutes, and immobilization biotechnology, 40(5), 354-361 (2012-05-01)
In this study, a novel amperometric glucose biosensor was developed by immobilizing glucose oxidase (GOX) by cross-linking via glutaraldehyde on electrochemically polymerized polypyrrole-poly(vinyl sulphonate) (PPy-PVS) films on the surface of a platinum (Pt) electrode. Electropolymerization of pyrrole and poly(vinyl sulphonate)
Halit Arslan et al.
Artificial cells, blood substitutes, and immobilization biotechnology, 39(5), 341-345 (2011-09-09)
Abstract: In this paper, a novel amperometric phenol biosensor with immobilization of polyphenol oxidase (tyrosinase) on electrochemically polymerized polypyrrole-polyvinylsulphonate (PPy-PVS) film has been accomplished via the entrapment technique on the surface of a platinum electrode. The amperometric determination is based
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service