All Photos(2)
About This Item
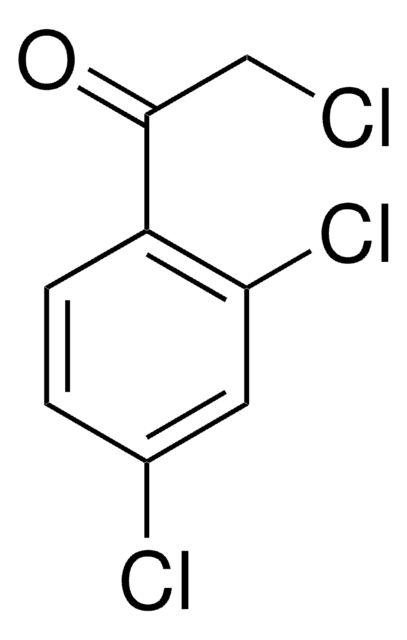
Linear Formula:
ClC6H4COCH2Cl
CAS Number:
Molecular Weight:
189.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
bp
270 °C (lit.)
mp
100-102 °C (lit.)
functional group
chloro
ketone
SMILES string
ClCC(=O)c1ccc(Cl)cc1
InChI
1S/C8H6Cl2O/c9-5-8(11)6-1-3-7(10)4-2-6/h1-4H,5H2
InChI key
FWDFNLVLIXAOMX-UHFFFAOYSA-N
General description
2,4′-Dichloroacetophenone is a substituted α-chloro aromatic ketone.2 Its 35/37Cl WURST-QCPMG NMR spectra has been investigated.
Application
2,4′-Dichloroacetophenone (4-chlorophenacyl chloride) may be used in the synthesis of chiral chlorohydrin by asymmetric reduction., It may be used as an alkylating agent in the Williamson reaction of 7-hydroxycoumarins to form substituted oxoethers.
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Modified coumarins. 10. Synthesis of substituted 2-(7-oxofuro [3, 2-g] chromen-6-yl) acetic acids.
Nagorichna IV, et al.
Chemistry of Natural Compounds, 39(3), 253-261 (2003)
Noriyuki Kizaki et al.
Bioscience, biotechnology, and biochemistry, 69(1), 79-86 (2005-01-25)
Optically active styrene oxide derivatives are versatile chiral building blocks. Stereoselective reduction of phenacyl halide to chiral 2-halo-1-phenylethanol is the key reaction of the most economical synthetic route. Rhodotorula glutinis var. dairenensis IFO415 was discovered on screening as a potent
Takeshi Ohkuma et al.
Organic letters, 9(2), 255-257 (2007-01-16)
Asymmetric hydrogenation of various alpha-chloro aromatic ketones with Ru(OTf)(TsDPEN)(eta6-arene) (TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine) produces the chiral chlorohydrins in up to 98% ee. This reaction can be conducted even on a 206-g scale. The hydrogenation of an alpha-chloro ketone with a phenol
Direct Investigation of Covalently Bound Chlorine in Organic Compounds by Solid-State 35Cl NMR Spectroscopy and Exact Spectral Line-Shape Simulations.
Perras FA and Bryce DL.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 124(11), 4303-4306 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service