All Photos(2)
About This Item
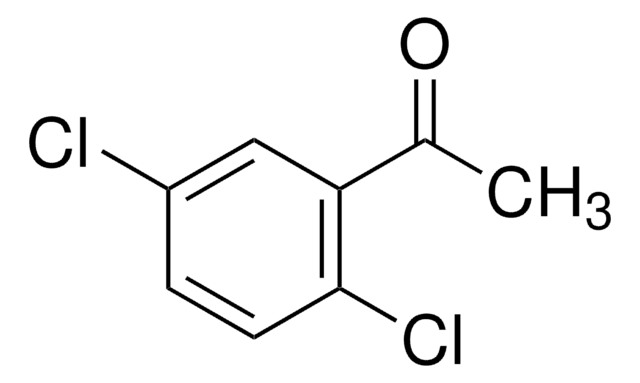
Linear Formula:
Cl2C6H3COCH3
CAS Number:
Molecular Weight:
189.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
bp
135 °C/12 mmHg (lit.)
mp
72-74 °C (lit.)
SMILES string
CC(=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C8H6Cl2O/c1-5(11)6-2-3-7(9)8(10)4-6/h2-4H,1H3
InChI key
WBPAOUHWPONFEQ-UHFFFAOYSA-N
Application
3′,4′-Dichloroacetophenone (3,4-Dichloroacetophenone) was used in the synthesis of substituted aryl aminothiazoles.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hamid Latif Siddiqui et al.
Chemical & pharmaceutical bulletin, 55(7), 1014-1017 (2007-07-03)
Different dimeric disulfides, having the basic skeleton of bis[2-amino-4-phenyl-5-thiazolyl] disulfides were synthesized in a straightforward manner from acetophenones. 2-Amino-4-phenyl-1,3-thiazoles were prepared by the reaction of thiourea with substituted acetophenones in the presence of iodine which were then converted to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1-[4-(3,4-Dichlorophenyl)phenyl]ethan-1-one](/deepweb/assets/sigmaaldrich/product/structures/246/776/d84e3b15-81c8-4f8b-a1ad-f663bc2d1fdf/640/d84e3b15-81c8-4f8b-a1ad-f663bc2d1fdf.png)