If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
221015
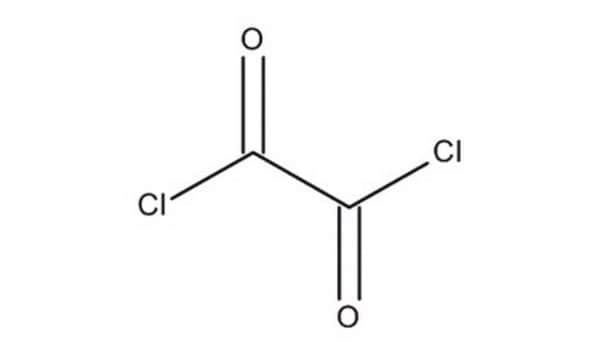
Oxalyl chloride
ReagentPlus®, ≥99%
Synonym(s):
Ethanedioyl dichloride
Select a Size
About This Item
Recommended Products
vapor density
4.4 (vs air)
Quality Level
vapor pressure
150 mmHg ( 20 °C)
product line
ReagentPlus®
assay
≥99%
form
liquid
reaction suitability
reagent type: oxidant
impurities
<10 ppb Heavy metals
color
APHA: 0-150
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Preparation of Mosher′s acid chloride by reacting with Mosher′s acid in the presence of DMF.[2]
- Activation of dimethyl sulfoxide for use in the oxidation of long-chain alcohols to carbonyls.[3]
- Activation of α-keto carboxylic acids and N-heterocyclic carboxylic acids for alkynylation to form ynediones and N-heterocyclic ynones, respectively.[4]
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
Packaging
Legal Information
accessory
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 1
flash_point_f
51.8 °F - closed cup
flash_point_c
11.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service