Stock solutions of this product may be stored at -20 °C for up to three months in aliquots. Stability data has not been collected for stock solutions at 2-8°C. This product does not need to be protected from light as it is not light-sensitive. Please see the link below to review the product datasheet for additional information:
https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/385/900/c9911pis.pdf
C9911
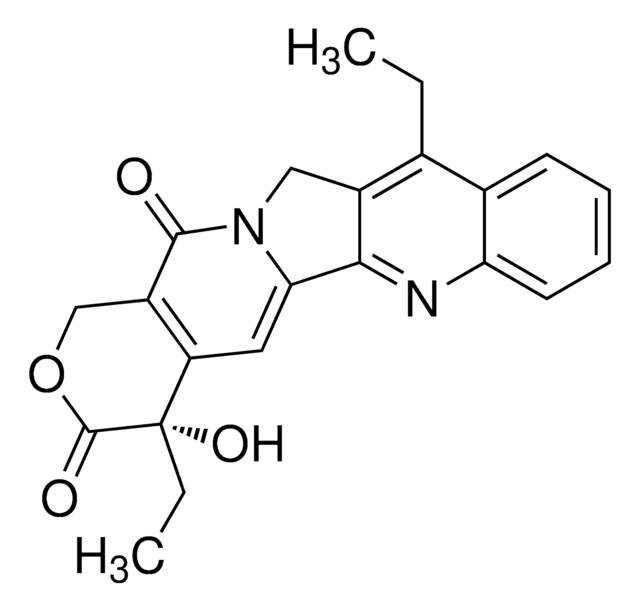
(S)-(+)-Camptothecin
≥90% (HPLC), powder, DNA topoisomerase I inhibitor
Sinónimos:
(S)-4-Ethyl-4-hydroxy-1H-pyrano-[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
Nombre del producto
(S)-(+)-Camptothecin, ≥90% (HPLC), powder
Ensayo
≥90% (HPLC)
Formulario
powder
mp
260 °C (dec.) (lit.)
solubilidad
chloroform/methanol (4:1): 5 mg/mL
espectro de actividad antibiótica
neoplastics
Modo de acción
DNA synthesis | interferes
enzyme | inhibits
temp. de almacenamiento
2-8°C
cadena SMILES
CC[C@@]1(O)C(=O)OCC2=C1C=C3N(Cc4cc5ccccc5nc34)C2=O
InChI
1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
Clave InChI
VSJKWCGYPAHWDS-FQEVSTJZSA-N
Información sobre el gen
human ... TOP1(7150)
mouse ... Prkca(18750)
rat ... Sstr2(54305)
¿Está buscando productos similares? Visita Guía de comparación de productos
Acciones bioquímicas o fisiológicas
Características y beneficios
Otras notas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Muta. 1B
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We presents an article on Autophagy in Cancer Promotes Therapeutic Resistance
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
-
How long can (S)-(+)-camptothecin C9911 be stored at 2-8°C? I am planning to make a 10 mg/ml stock using DMSO; should it be protected from light? Is it light-sensitive?
1 answer-
Helpful?
-
-
How long can (S)-(+)-camptothecin C9911 be stored at 2-8°C? Should it be protected from light? Is it light-sensitive?
1 answer-
Stock solutions of this product may be stored at -20 °C for up to three months in aliquots. Stability data has not been collected for stock solutions at 2-8°C. This product does not need to be protected from light as it is not light-sensitive. Please see the link below to review the product datasheet for additional information:
https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/385/900/c9911pis.pdfHelpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is Product C9911, (S)-(+)-Camptothecin, soluble in?
1 answer-
This product is soluble in DMSO (10 mg/mL). At higher concentrations, heating is required for the product to dissolve completely (approximately 10 minutes at 95°C), but some precipitation occurs upon cooling to room temperature. It is also soluble in 1 N NaOH (50 mg/mL) and methanol (50 mg/mL).
Helpful?
-
-
What is the extinction coefficient of Product C9911, (S)-(+)-Camptothecin?
1 answer-
The millimolar extinction coefficients (EmM) at various wavelengths are 37.3 (220 nm); 29.2 (254 nm); 4.9 (290 nm); 19.9 (370 nm).
Helpful?
-
-
What is the solution stability of Product C9911, (S)-(+)-Camptothecin?
1 answer-
Stock solutions of camptothecin in DMSO can be stored at -20°C for up to 3 months.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico