M8703
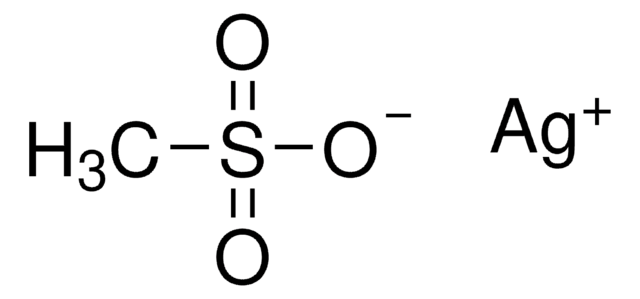
Silver methanesulfonate
98%
Synonym(s):
Methanesulfonic acid silver salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
AgSO3CH3
CAS Number:
Molecular Weight:
202.97
MDL number:
PubChem Substance ID:
Recommended Products
assay
98%
mp
252-256 °C (lit.)
SMILES string
[Ag+].CS([O-])(=O)=O
InChI
1S/CH4O3S.Ag/c1-5(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
MLKQJVFHEUORBO-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst for:
- Heterocyclization reactions
- CO2-mediated rearrangement of propargyl alcohols for the synthesis of a,ß-unsaturated ketones and esters
Precursor to alkyl methanesulfonate derivatives useful as alkylating agents for aromatic compounds.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tetrahedron Letters, 22, 537-537 (1981)
Tsung-Ming Shih et al.
Chemico-biological interactions, 187(1-3), 207-214 (2010-03-13)
This study compared the ability of nine oximes (HI-6, HLö7, MMB-4, TMB-4, carboxime, ICD585, ICD692, ICD3805, and 2-PAM) to reactivate in vivo cholinesterase (ChE) in blood, brain, and peripheral tissues in guinea pigs intoxicated by one of four organophosphorus nerve
Xiangke Chen et al.
The journal of physical chemistry. B, 114(47), 15546-15553 (2010-11-11)
The molecular organization at the aqueous dimethyl sulfoxide (DMSO) and methanesulfonic acid (MSA) surfaces was investigated using vibrational sum frequency generation (VSFG) spectroscopy and molecular dynamics (MD) simulation. The molecular orientation of surface DMSO and MSA is deduced based on
Statistically sound calibration curves for chromatographic methods involving negative response data.
L E Vanatta et al.
Journal of chromatographic science, 49(8), 610-611 (2011-08-24)
A statistical procedure is presented for calibrating chromatographic methods that generate negative chromatographic peaks. The technique is illustrated via data collected during the ion-chromatographic analysis of the ammonium ion in methansulfonic-acid solutions. A peak-integration protocol is explained and subsequent regression
Neal W Sach et al.
Organic letters, 14(15), 3886-3889 (2012-07-18)
A general synthesis of aryl ethers from primary and secondary alcohols and aryl mesylates is presented. The reaction proceeds via a sulfonyl-transfer mechanism. In this paper, we compare the sulfonyl transfer reaction to Mitsunobu ether formation. The reaction can be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service