402869
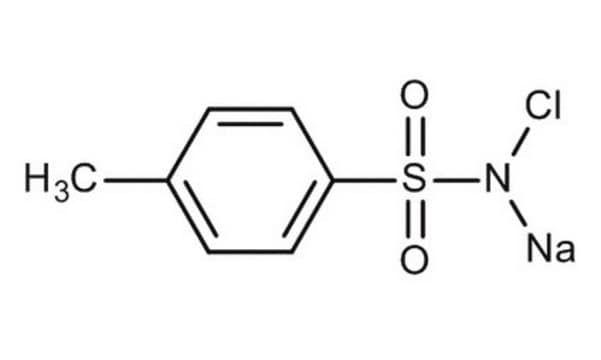
Chloramine T trihydrate
ACS reagent, 98%
Synonym(s):
N-Chloro-p-toluenesulfonamide sodium salt
About This Item
Recommended Products
grade
ACS reagent
Quality Level
assay
98%
98.0-103.0% (ACS specification)
form
powder
reaction suitability
reagent type: oxidant
impurities
<1.5% insolubles (alcohol)
pH
8.0-10.0 (25 °C, 5%)
mp
167-170 °C (lit.)
solubility
H2O: passes test
suitability
passes test for determination of bromide
SMILES string
O.O.O.Cc1ccc(cc1)S(=O)(=O)N([Na])Cl
InChI
1S/C7H7ClNO2S.Na.3H2O/c1-6-2-4-7(5-3-6)12(10,11)9-8;;;;/h2-5H,1H3;;3*1H2/q-1;+1;;;
InChI key
NZYOAGBNMCVQIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
application
It may also be used in the synthesis of the following compounds:
- Nitrile imines by the oxidative dehydrogenation of N-(nitrobenzyl)-imidazole aldehyde hydrazine.
- Mono-N-tosylated-1,2-diamines.
- 3,5-Disubstituted isoxazoles.
- Fused 3,6-disubstituted triazolothiadiazoles by the oxidative cyclization of N-heteroaryl-substituted hydrazones.
Packaging
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B
supp_hazards
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 2
flash_point_f
377.6 °F - closed cup
flash_point_c
192 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service