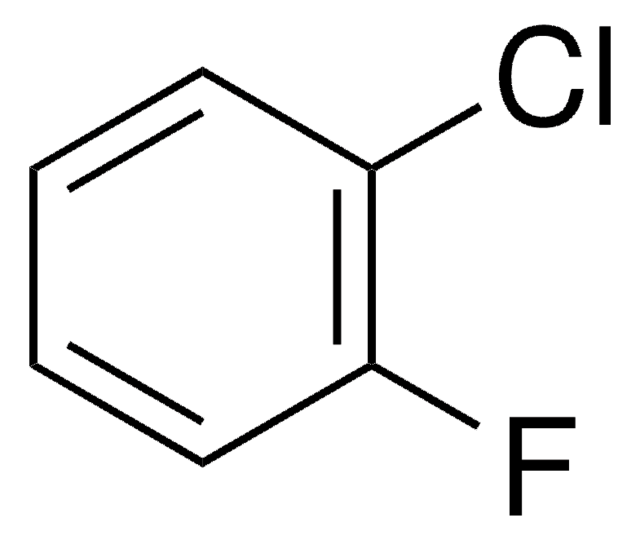
D102008
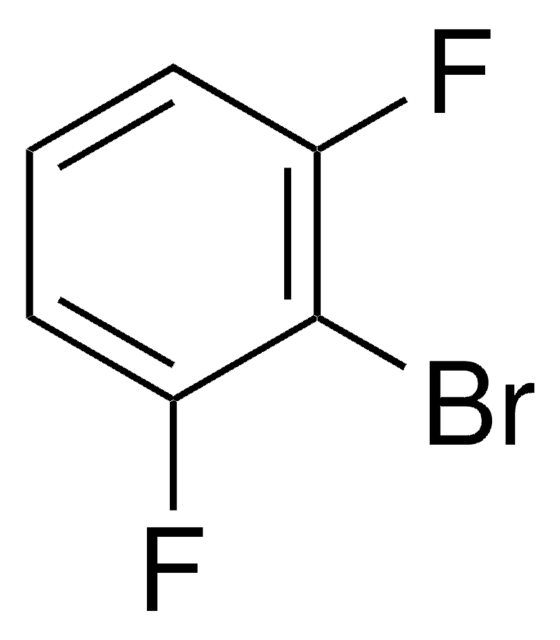
1,3-Difluorobenzene
≥99%
Synonym(s):
1,3-Bisfluorobenzene, 1,3-Difluorobenzene, 2,4-Difluorobenzene, m-Difluorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H4F2
CAS Number:
Molecular Weight:
114.09
Beilstein/REAXYS Number:
1904537
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥99%
form
liquid
refractive index
n20/D 1.438 (lit.)
bp
82 °C (lit.)
density
1.163 g/mL at 25 °C (lit.)
SMILES string
Fc1cccc(F)c1
InChI
1S/C6H4F2/c7-5-2-1-3-6(8)4-5/h1-4H
InChI key
UEMGWPRHOOEKTA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Gala et al.
Journal of pharmaceutical sciences, 81(12), 1199-1203 (1992-12-01)
Alpha-Hydroxyaryl ketones such as 2-hydroxypropiophenone and 1-(2,4-difluorophenyl)-2-hydroxy-1-propanone, the key intermediates in the preparation of antifungal agents, decompose into oxidized, rearranged, and condensed products. These products were isolated and characterized. The possible mechanisms for the formation of the products are discussed.
Aujin Kim et al.
The Journal of organic chemistry, 71(5), 2170-2172 (2006-02-25)
A short, high-yielding synthesis of differentially substituted resorcinol derivatives has been developed that utilizes 1,3-difluorobenzene as the starting material and employs sequential nucleophilic aromatic substitution (S(N)Ar) reactions to generate desymmetrized products. The scope and limitations of the second S(N)Ar reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service