906190
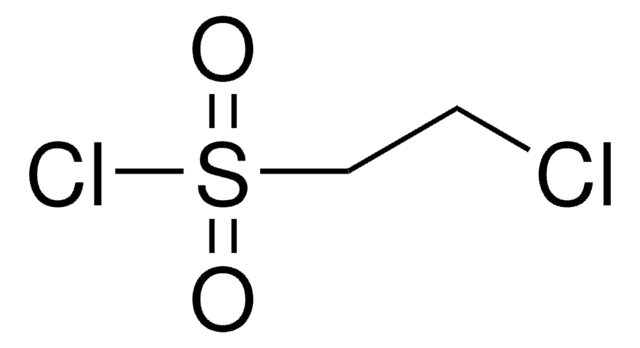
1,2-Dibromoethane-1-sulfonyl fluoride
Synonym(s):
DESF, SuFEx hub, SuFEx-able plugin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3Br2FO2S
CAS Number:
Molecular Weight:
269.92
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
liquid
reaction suitability
reaction type: click chemistry
Application
1,2-Dibromoethane-1-sulfonyl fluoride (DESF) is a bench-stable precursor to 1-bromoethene-1-sulfonyl fluoride (BESF), a new and robust connective hub for the Sulfur (VI) fluoride exchange (SuFEx) click reaction. BESF offers similar routes as ethenesulfonyl fluoride (ESF, cat# 746959) but with additional reactivity due to the embedded bromo group.
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jing Leng et al.
Chemical communications (Cambridge, England), 54(35), 4477-4480 (2018-04-17)
A new fluorosulfonylation reagent 1-bromoethene-1-sulfonyl fluoride was developed (1-Br-ESF). This unique reagent possesses three addressable handles (vinyl, bromide, and sulfonyl fluoride) and has great potential to function as a tris-electrophile and as a sulfur(vi) fluoride exchange (SuFEx) clickable material to
Christopher J Smedley et al.
Chemical communications (Cambridge, England), 54(47), 6020-6023 (2018-05-26)
We demonstrate 1,2-dibromoethane-1-sulfonyl fluoride (DESF) as a bench-stable and readily accessible precursor to the robust SuFEx connector, 1-bromoethene-1-sulfonyl fluoride (BESF). The in situ generation of BESF from DESF opens up several new reaction profiles, including application in the syntheses of
Joice Thomas et al.
Organic letters, 20(13), 3749-3752 (2018-06-16)
A regioselective metal-free preparation of 4-fluorosulfonyl 1,2,3-triazoles from organic azides and a hitherto underexplored bromovinylsulfonyl fluoride building block is described. This reaction is very general and was extended to the synthesis of various sulfonates, sulfonamides, and sulfonic acid derivatives of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service