
自1997年Ellman將其作為手性氨等價物推出以來,1 對映純2-甲基-2-丙磺酰胺(tert-butanesulfinamide)已被證明是一種多用途手性助劑,並在學術界和工業界得到了廣泛的應用。在溫和的條件下,tert-丁烷亞磺酰胺與醛和酮進行縮合,並提供高產率的 tert-丁烷亞磺酰亞胺。tert丁烷亞磺酰基可激活這些亞胺,使其與多種不同類型的親核劑相加,並作為強大的手性導向基團,提供具有普遍高非對映選擇性的產品。其後,在溫和的條件下除去丁烷亞磺酰基,就可以乾淨地提供胺產品。
這些 tert丁烷亞磺酰亞胺已被用作許多多功能構建砌塊不對稱合成的中間體
。sup>2 包括 合成- 和 反- 1,2-或 1,3-氨基醇,3,4 α支鏈胺和 α,α二支鏈胺,5及 α- 或 β- 氨基酸及酯6,7 (方案 1)。多位研究人員已利用化學性,合成抗生素、生物活性化合物和其他複雜的天然產品。8 此外, tert-丁烷亞磺酰亞胺已被用於合成不對稱配體,9 並且在少數情況下,作為手性成分出現。10

方案 1.使用叔丁基亞磺酰亞胺不對稱合成多種多用途構建塊
最近, tert-丁烷亞磺酰亞胺被用於合成手性雜環。一些研究小組通過一個普通的 tert-丁烷亞磺酰亞胺中間體(方案 2)合成了手性氮丙啶。Morton 及其合作者使用三甲基碘化锍合成手性氮丙啶,具有良好的產率和非對映選擇性。11a Chemla和Ferreira將外消旋的allenylzinc底物與多種 t丁烷亞胺反應,以良好的產率獲得非對映和對映源純的反式乙炔基氮丙啶化合物。11b
。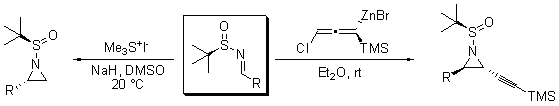
方案 2.透過一般叔丁基亞氨基磺酰基中間體合成手性氮丙啶
此外,Dondas 和 De Kimpe 利用常用的外消旋 tert丁烷亞磺胺(方案 3)設計了一條通往吡咯烷和哌啶的高效路線,該亞磺胺通過 NaBH4 還原即可輕鬆實現。12 他們的合成突出了一鍋級聯的環化和碎片化,這使得環化產物的產率和純度非常高。

方案 3.使用普通外消旋叔丁基亞磺酸胺的吡咯烷和哌啶
Ellman的研究小組也報導了手性雜環的合成。在他們合成 1,3-氨基醇工作的延伸中,4a Ellman 進行了 (-)-halosaline 和 (-)-8-epihalosaline 的不對稱合成 (Scheme 4)。
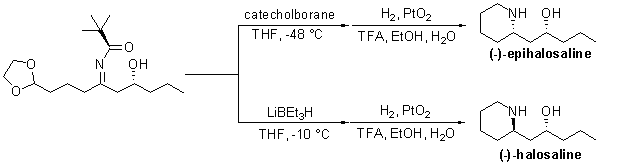
方案 4.(-)-哈洛沙林和(-)-8-表哈洛沙林的不對稱合成
最近的另一份報告描述了手性 tert丁烷亞磺酰亞胺在合成吡咯烷生物鹼 SC-53116 (方案 5)中的分子間自縮合。13 在稍後的報告中,Ellman 證明了手性 2-取代的吡咯烷(方案 6)的簡易合成,過程具有高產率和非對映選擇性。

方案 5.手性叔丁基亞磺酰亞胺自縮形成吡咯烷生物鹼

方案 6.手性 2-取代吡咯烷的簡易合成
我們很高興能為您的研究提供對映体和外消旋體兩種形式的多用途實用助劑。
參考資料
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?