推薦產品
等級
pharmaceutical primary standard
API 家族
tetracaine
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
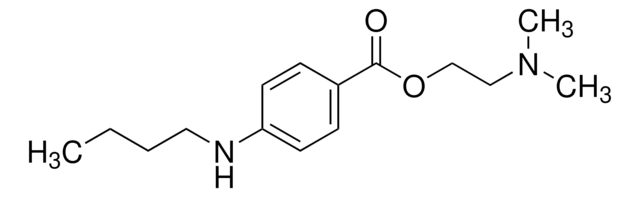
Cl.CCCCNc1ccc(cc1)C(=O)OCCN(C)C
InChI
1S/C15H24N2O2.ClH/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3;/h6-9,16H,4-5,10-12H2,1-3H3;1H
InChI 密鑰
PPWHTZKZQNXVAE-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
尋找類似的產品? 前往 產品比較指南
一般說明
應用
- Benzocaine, Butamben, and Tetracaine Hydrochloride Gel
- Benzocaine, Butamben, and Tetracaine Hydrochloride Ointment
- Benzocaine, Butamben, and Tetracaine Hydrochloride Topical Aerosol®
- Benzocaine, Butamben, and Tetracaine Hydrochloride Topical Solution
- Lidocaine, Racepinephrine, and Tetracaine Hydrochlorides Compounded Topical Gel
- Neomycin Sulfate, Isoflupredone Acetate, and Tetracaine Hydrochloride Ointment
- Neomycin Sulfate, Isoflupredone Acetate, and Tetracaine Hydrochloride Topical Powder
生化/生理作用
分析報告
其他說明
法律資訊
相關產品
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務