推薦產品
等級
pharmaceutical primary standard
API 家族
levetiracetam
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
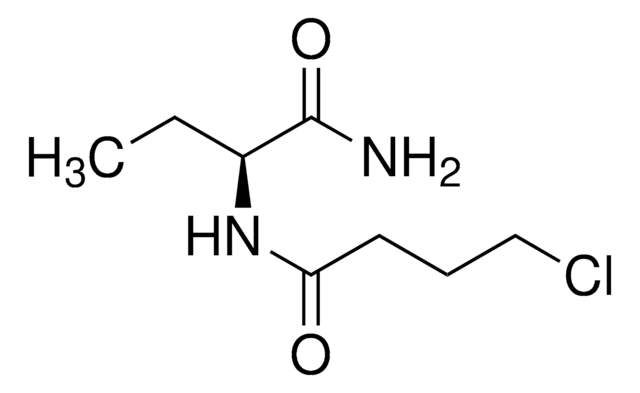
CC[C@H](N1CCCC1=O)C(N)=O
InChI
1S/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/m0/s1
InChI 密鑰
HPHUVLMMVZITSG-LURJTMIESA-N
基因資訊
human ... CACNA1B(774) , SV2A(9900)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Levetiracetam USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
生化/生理作用
左乙拉西坦是一种具有抗癫痫活性的吡咯烷。左乙拉西坦的立体选择性结合仅限于中枢神经系统的突触质膜,而在周围组织中不发生立体选择性结合。左乙拉西坦在不影响正常神经元兴奋性的情况下抑制簇状放电,这表明它可以选择性地阻止癫痫样簇状放电的超同步和发作活动的传播。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Benefit of combination therapy in epilepsy: a review of the preclinical evidence with levetiracetam.
Rafal M Kaminski et al.
Epilepsia, 50(3), 387-397 (2008-07-17)
Levetiracetam (Keppra) is an antiepileptic drug (AED) characterized by a novel mechanism of action, unique profile of activity in seizure models, and broad-spectrum clinical efficacy. The present report critically reviews several preclinical studies focused on combination therapy with levetiracetam and
J Rösche et al.
Fortschritte der Neurologie-Psychiatrie, 81(1), 21-27 (2012-08-15)
Non-convulsive status epilepticus and epilepsia partialis continua are common epileptic conditions for which straightforward recommendations based on controlled randomised trials for treatment of therapy refractory courses are lacking. Therefore in these conditions sometimes antiepileptic drugs that are not approved by
Katherine A Lyseng-Williamson
Drugs, 71(4), 489-514 (2011-03-15)
Levetiracetam (Keppra®, E Keppra®) is an established second-generation antiepileptic drug (AED). Worldwide, levetiracetam is most commonly approved as adjunctive treatment of partial onset seizures with or without secondary generalization; other approved indications include monotherapy treatment of partial onset seizures with
Justin B Usery et al.
Journal of neuro-oncology, 99(2), 251-260 (2010-02-11)
To determine the safety and tolerability of IV and oral levetiracetam monotherapy for seizures in brain tumor patients following resection. Brain tumor patients undergoing neurosurgery with >or=1 seizure within the preceding month prior to surgery were enrolled to receive intravenous
Vincenzo Belcastro et al.
Brain & development, 33(4), 289-293 (2010-07-16)
Several new antiepileptic drugs (AEDs) have been introduced for clinical use recently. These new AEDs, like the classic AEDs, target multiple cellular sites both pre- and postsynaptically. The use of AEDs as a possible neuroprotective strategy in brain ischemia is
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務