推薦產品
等級
pharmaceutical primary standard
API 家族
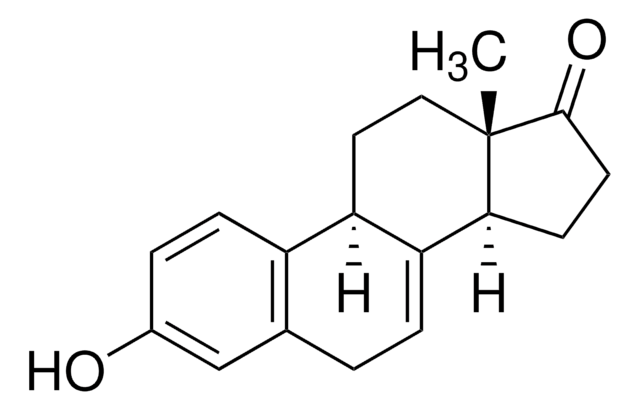
estrone
製造商/商標名
USP
mp
258-260 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CCC2=O
InChI
1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChI 密鑰
DNXHEGUUPJUMQT-CBZIJGRNSA-N
基因資訊
human ... ESR1(2099)
尋找類似的產品? 前往 產品比較指南
應用
Estrone USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Conjugated Estrogens
- Conjugated Estrogens Tablets
- Esterified Estrogens
- Esterified Estrogens Tablets
- Estradiol
- Estradiol and Norethindrone Acetate Tablets
- Estradiol Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Carc. 2 - Lact. - Repr. 1A
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Robert E Nelson et al.
Clinical chemistry, 50(2), 373-384 (2003-12-06)
Estradiol (E2) and estrone (E1) measurements form an integral part of the assessment of female reproductive function and have expanding roles in other fields. However, many E1 and E2 immunoassays have limited functional sensitivity, suffer from cross-reactivity, and display poor
Fluorometric determination of plasma 11-hydroxycorticosteroids. II. Studies on the specificity of the method.
L E Mejer et al.
Clinical chemistry, 19(7), 718-724 (1973-07-01)
Shanshan Zhao et al.
Breast cancer research : BCR, 16(2), R30-R30 (2014-03-29)
Paradoxically, a breast cancer risk reduction with conjugated equine estrogens (CEE) and a risk elevation with CEE plus medroxyprogesterone acetate (CEE + MPA) were observed in the Women's Health Initiative (WHI) randomized controlled trials. The effects of hormone therapy on serum sex
Eva Fetter et al.
Aquatic toxicology (Amsterdam, Netherlands), 154, 221-229 (2014-06-14)
Xenoestrogens may persist in the environment by binding to sediments or suspended particulate matter serving as long-term reservoir and source of exposure, particularly for organisms living in or in contact with sediments. In this study, we present for the first
Xavier Remesar et al.
Medicinal research reviews, 32(6), 1263-1291 (2011-02-03)
Oleoyl-estrone (OE) is a powerful slimming agent that is also present in plasma and adipose tissue, where it is synthesized. It acts through the formation of a derivative W. OE effects (and W levels) are proportional to the dose. OE
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務