推薦產品
等級
pharmaceutical primary standard
API 家族
cilostazol
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
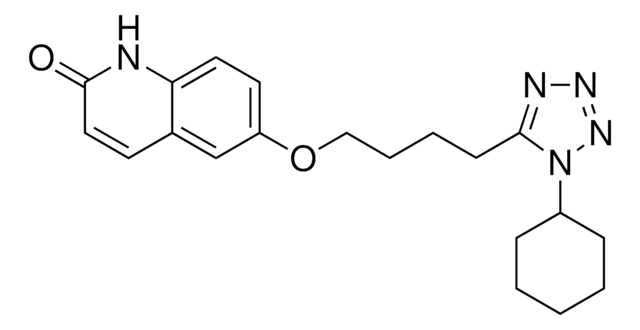
O=C1CCc2cc(OCCCCc3nnnn3C4CCCCC4)ccc2N1
InChI
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
InChI 密鑰
RRGUKTPIGVIEKM-UHFFFAOYSA-N
基因資訊
human ... PDE3A(5139)
尋找類似的產品? 前往 產品比較指南
一般說明
Cilostazol is a potent cyclic nucleotide phosphodiesterase inhibitor. It is mainly used as antiplatelet agent.
應用
Cilostazol USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Cilostazol Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
S Takahashi et al.
Journal of cardiovascular pharmacology, 20(6), 900-906 (1992-12-01)
Cilostazol, a cyclic AMP phosphodiesterase inhibitor, has been used as an antiplatelet agent. In the present study, we investigated the in vitro effect of cilostazol on DNA synthesis in rat aortic arterial smooth muscle cells (SMCs) in culture stimulated with
Sayuri N Friedland et al.
The American journal of cardiology, 109(10), 1397-1404 (2012-03-03)
Cilostazol is a generic drug with antiplatelet and antiproliferative effects. It is unclear whether adding cilostazol to standard dual antiplatelet therapy (aspirin and clopidogrel) after percutaneous coronary intervention reduces restenosis and improves the outcomes. We, therefore, conducted a systematic review
James J Dinicolantonio et al.
The American journal of cardiology, 112(8), 1230-1234 (2013-07-06)
Aspirin is the most widely prescribed antiplatelet agent for the secondary prevention of stroke. Cilostazol, an antiplatelet and vasodilating agent, has shown promise for the secondary prevention of stroke. A systematic review and meta-analysis of randomized controlled trials using Ovid
Kelly C Rogers et al.
The Annals of pharmacotherapy, 46(6), 839-850 (2012-06-07)
To evaluate the addition of cilostazol to standard dual antiplatelet therapy (DAT) with aspirin and clopidogrel in patients receiving coronary stenting. Relevant information was identified through a search of MEDLINE (1966-November 2011), International Pharmaceutical Abstracts (1960-2011), and Cochrane Databases (publications
Natnicha Kanlop et al.
Journal of cardiovascular medicine (Hagerstown, Md.), 12(2), 88-95 (2011-01-05)
Cilostazol is a selective phosphodiesterase 3 (PDE3) inhibitor approved by the Food and Drug Administration for treatment of intermittent claudication. It has also been used in bradyarrhythmic patients to increase heart rates. Recently, cilostazol has been shown to prevent ventricular
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務