推薦產品
等級
analytical standard
品質等級
CofA
current certificate can be downloaded
包裝
ampule of 25 mg
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
177-180 °C (lit.)
應用
cleaning products
cosmetics
environmental
food and beverages
personal care
形式
neat
儲存溫度
2-30°C
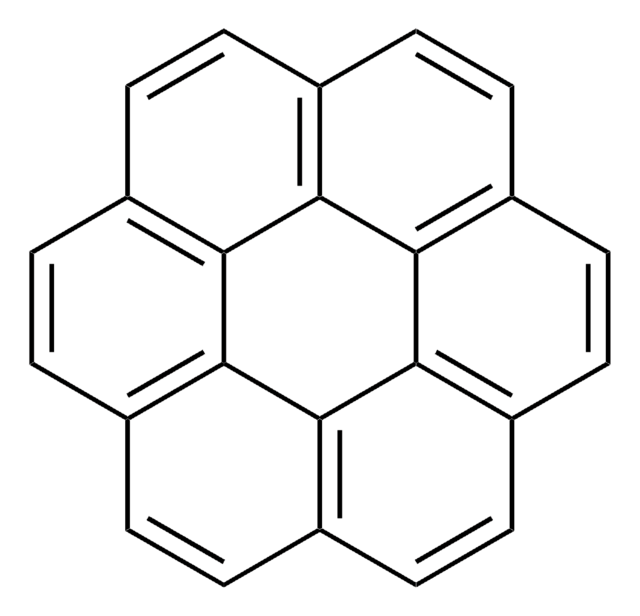
SMILES 字串
c1ccc2c(c1)c3cccc4ccc5cccc2c5c34
InChI
1S/C20H12/c1-2-8-16-15(7-1)17-9-3-5-13-11-12-14-6-4-10-18(16)20(14)19(13)17/h1-12H
InChI 密鑰
TXVHTIQJNYSSKO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
苯并[e]芘是一种五环芳烃污染物。 它是强效致癌物苯并[a]芘的结构异构体。
應用
有关合适仪器技术的更多信息,请参考产品′s分析证书。如需进一步支持,请联系技术服务。
訊號詞
Danger
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Formation of sulfate and glucoside conjugates of benzo [e] pyrene by Cunninghamella elegans
Pothuluri.V.J, et al.
Applied Microbiology and Biotechnology, 45, 677-683 (1996)
Photochemical transformations of benzo [e] pyrene in solution and adsorbed on silica gel and alumina surfaces
Fioressi S and Arce R
Environmental Science & Technology, 39, 3646-3655 (2005)
Daniëlle M J Curfs et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 19(10), 1290-1292 (2005-06-09)
Although it has been demonstrated that carcinogenic environmental polycyclic aromatic hydrocarbons (PAHs) cause progression of atherosclerosis, the underlying mechanism remains unclear. In the present study, we aimed to investigate whether DNA binding events are critically involved in the progression of
Joris J H Haftka et al.
Environmental toxicology and chemistry, 27(7), 1526-1532 (2008-02-12)
The uptake kinetics of fluorene, phenanthrene, fluoranthene, pyrene, and benzo[e]pyrene by solid-phase microextraction fibers was studied in the presence of dissolved organic matter (DOM) obtained from sediment pore water and resulted in increased fiber absorption and desorption rate coefficients. Compared
Gabriela L Borosky et al.
Organic & biomolecular chemistry, 5(14), 2234-2242 (2007-07-05)
A DFT study aimed at understanding structure-reactivity relationships and fluorine substitution effects on carbocation stability in benzo[a]pyrene (BaP), benzo[e]pyrene (BeP), and aza-benzo[a]pyrene (aza-BaP) derivatives are reported. The relative energies of the resulting carbocations are examined and compared, taking into account
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![苯并[a]芘 ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)
![苯并[e]芘 98%](/deepweb/assets/sigmaaldrich/product/structures/162/859/cd1f8e1f-2539-4f36-be04-8bad9d301215/640/cd1f8e1f-2539-4f36-be04-8bad9d301215.png)
![苯并[a]芘-d12 98 atom % D](/deepweb/assets/sigmaaldrich/product/structures/962/892/b867e1bb-083c-4337-b499-36eae87f40ad/640/b867e1bb-083c-4337-b499-36eae87f40ad.png)

![苯并 [ a ] 蒽 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/351/486/b3ddf157-a732-4ef8-83f0-c70a53404cb2/640/b3ddf157-a732-4ef8-83f0-c70a53404cb2.png)
![苯并[b]荧蒽 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)



![苯并[ghi]苝 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)