推薦產品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
2-8°C
SMILES 字串
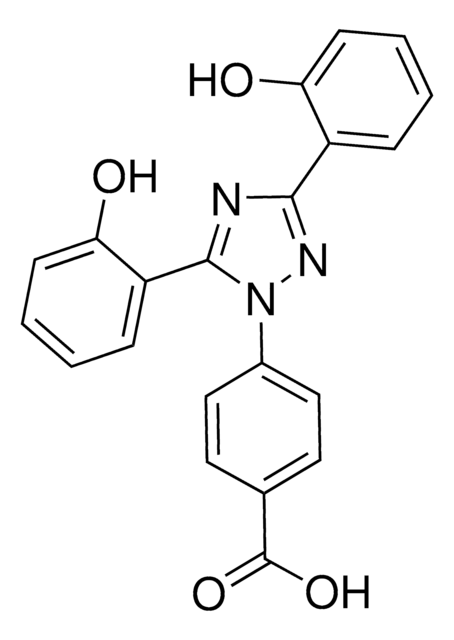
N2(N\C(=C4\C=CC=CC\4=O)\N\C\2=C3\C=CC=CC\3=O)c1ccc(cc1)C(=O)O
InChI
1S/C21H15N3O4/c25-17-7-3-1-5-15(17)19-22-20(16-6-2-4-8-18(16)26)24(23-19)14-11-9-13(10-12-14)21(27)28/h1-12,22-23H,(H,27,28)/b19-15-,20-16+
InChI 密鑰
FMSOAWSKCWYLBB-VBGLAJCLSA-N
尋找類似的產品? 前往 產品比較指南
應用
Deferasirox has been used as an iron chelator to test its effect on clofazimine mediated growth inhibition and rescue in Salmonella typhimurium.[1]
生化/生理作用
Deferasirox belongs to the N-substituted bis-hydroxyphenyl-triazole family of tridentate iron chelators.[2]
Deferasirox is an orally available iron chelator used clinically for reduction of chronic iron overload in diseases such as β-thalassemia.
Orally available iron chelator
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
Kangna Cao et al.
European journal of clinical pharmacology, 76(1), 51-59 (2019-11-05)
Our aim was to evaluate the influence of genetic polymorphisms involved in the metabolism and transportation of deferasirox on deferasirox pharmacokinetics in the Chinese population. Thirty-eight healthy Chinese subjects were administered with a single dose of 20 mg kg-1 deferasirox.
Goldie Y L Lui et al.
Oncotarget, 6(22), 18748-18779 (2015-07-01)
Newer and more potent therapies are urgently needed to effectively treat advanced cancers that have developed resistance and metastasized. One such strategy is to target cancer cell iron metabolism, which is altered compared to normal cells and may facilitate their
Juan Daniel Díaz-García et al.
Nature reviews. Nephrology, 10(10), 574-586 (2014-07-23)
In 2005, the oral iron chelator deferasirox was approved by the FDA for clinical use as a first-line therapy for blood-transfusion-related iron overload. Nephrotoxicity is the most serious and frequent adverse effect of deferasirox treatment. This nephrotoxicity can present as
Abbas Rahdar et al.
Life sciences, 270, 119146-119146 (2021-02-06)
Deferasirox (DFX) was formulated into oil-in-water microemulsions in the presence of pluronicto improve its oral bioavailability. The size of the DFX-loadedmicroemulsions system measured by dynamic light scattering (DLS) was about 9 nm. The anti-proliferative and anti-lipid peroxidation effects of DFX and
Wataru Goto et al.
BMC cancer, 20(1), 1215-1215 (2020-12-12)
Iron is required for the proliferation of cancer cells, and its depletion suppresses tumor growth. Eribulin mesylate (eribulin), a non-taxane microtubule inhibitor, disrupts the tumor microenvironment via vascular remodeling and obstruction of the epithelial-mesenchymal transition (EMT). Herein, we investigated the
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務